OGG synthesis was achieved through GG oxidation using sodium periodate (NaIO4). Structural confirmation was obtained through 1H nuclear magnetic resonance (1H NMR) and Fourier transform infrared spectroscopy (FTIR) analyses. The ¹H NMR spectrum revealed a characteristic aldehydic proton peak at 9.3 ppm (Figure S1(a)). The FTIR spectrum of pure GG (Figure S1(b)) revealed typical peaks at 3400 (O–H), 2910 (C–H), 1152 (C-O), and 1002 cm⁻¹ (glycosidic linkage of the GG pyranose ring). After the reaction of GG with NaIO4, a new peak was observed at 1727 cm–1, corresponding to the aldehyde symmetrical vibration. The aldehyde groups of the monomeric sugar units and the OH groups of the unoxidized ring residue can form a hemi acetal bond via the chemical reaction, which likely accounts for the relatively weak intensity of aldehyde group signals. Similarly, CMCS synthesis was verified through comparative 1HNMR and FTIR analyses of CS and CMCS. The 1HNMR spectrum showed characteristic peaks at 3.2–3.5 ppm for H-2 protons of carboxymethyl groups (Figure S1(c)). FTIR analysis revealed significant modifications in the chemical structure, where the characteristic amino group bending vibration at 1550 cm⁻¹ and C-O-C asymmetric stretching at 1242 cm⁻¹ in pristine CS were accompanied by a new absorption band at 1422 cm⁻¹ in CMCS, corresponding to the asymmetric stretching vibration of carboxylate groups (Figure S1(d)). These spectral features collectively demonstrate the successful introduction of carboxymethyl groups onto the chitosan backbone, confirming the synthesis of CMCS.
The fabrication of OGG/CMCS hydrogel involved simple mixing of OGG and CMCS solutions as the aldehyde group of OGG can form reversible Schiff base bonds with the amino group of CMCS. Hydrogels prepared by 1.5 wt% CMSC and varying concentrations of OGG (2.0 wt%, 1.5 wt%, and 1.0 wt%) were used to perform the in vitro physiochemical properties characterization. The microstructure of the hydrogels was characterized by observing freeze-dried samples using scanning electron microscopy (Figs. 2(a)–2(c)). Variations in the formulation of the hydrogel were found to impact its porosity. Specifically, the OGG/CMCS-2.0% hydrogel demonstrated smaller pore sizes and a denser network structure in comparison to the OGG/CMCS-1.5% and OGG/CMCS-1.0% (Figure S2).
Additionally, good homogeneity of the hydrogel plays a crucial role in enhancing its mechanical strength, while also providing a more suitable environment for cell adhesion and proliferation [18]. Consequently, dynamic rheology analysis was conducted on OGG/CMCS hydrogels with different formulations to further investigate these properties. For each hydrogel, its storage modulus (G’) exceeded the loss modulus (G”) consistently, signifying that the hydrogel was free-standing (Fig. 2(d)). The modulus–strain curves demonstrated that under strains less than 100%, the modulus of all hydrogels remained steady, with G’ surpassing G”, suggesting the network structure was stable within the range (Fig. 2(e)). As strain increased, the G’ declined, ultimately falling below G”, indicating the breakdown of the network structure. Besides, the OGG/CMCS-2.0% demonstrated the highest yielding modulus, whereas OGG/CMCS-1.0% exhibited the lowest yielding modulus, indicating a correlation between the mechanical properties and microscopic structures of the hydrogel. The compressive mechanical properties of the hydrogels were quantitatively evaluated, as illustrated in Figs. 2(f) and 2(g). A concentration-dependent enhancement in compressive strength was observed, with OGG/CMCS-2.0% exhibiting the highest maximum compressive strength, followed by OGG/CMCS-1.5%, while OGG/CMCS-1.0% demonstrated the lowest mechanical strength. This trend aligns with the rheological measurements.
Self-healing injectable hydrogels have extensive applications, including drug delivery and tissue regeneration [19], and offer a solution for the repair of an irregular-shaped corneal wound. Consequently, we assessed the rheological characterization of the OGG/CMCS hydrogels. All formulations exhibited pronounced shear-thinning behavior, with viscosity decreasing as shear rates increased under a constant 1% strain (Fig. 2(h)), indicating excellent injectability. This rheological property enables smooth extrusion through 25-gauge needles while maintaining structural integrity, as demonstrated in Fig. 2(i), without clogging. To confirm the self-healing capability of OGG/CMCS hydrogels, we fabricated two shell-shaped hydrogels containing pink and blue dyes (Figure S3). The hydrogels were cut with a blade into two half pieces, and one half containing pink dye was closely brought into contact with the one containing blue dye. After 2 hours, the two halves seamlessly healed into a unified structure, capable of supporting its weight under gravity (Figure S3). The healed unit also demonstrated the ability to withstand stretching up to almost twice its original length without rupturing (Fig. 2(j)). Moreover, continuous oscillatory step-strain measurements were conducted to test the rheological self-healing properties. During the test, the oscillatory strain transitioned from 1% over an 80-second interval to 400% over a 60-second interval, with a total of four cycles. When the strain reached 400%, G’ became less than G”, indicating hydrogel breakage. Yet, G’ promptly returned to the initial value as the strain reverted to 1%, surpassing G” (Fig. 2(k)). The observed self-healing capability facilitates both smooth injection through narrow-gauge needles and effective integration with corneal defects, enabling minimally invasive repair.
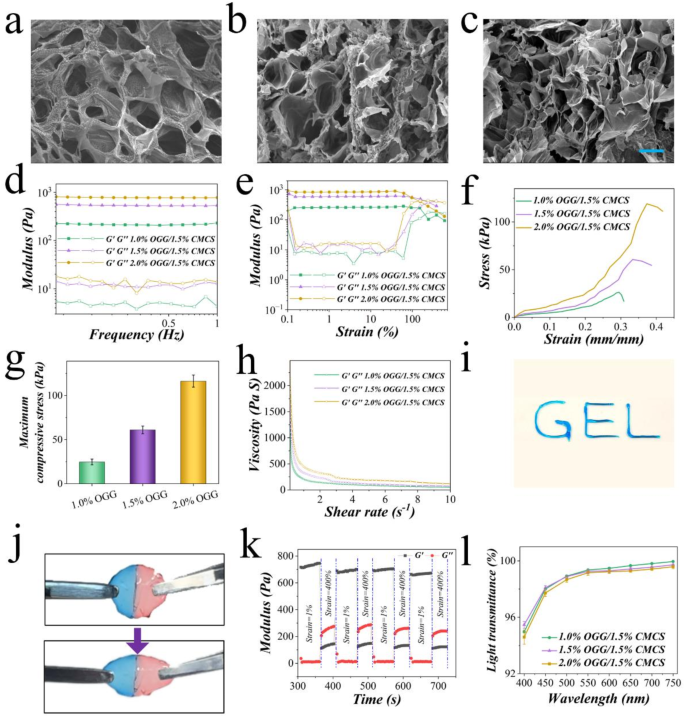
Fabrication and characterization of the OGG/CMCS hydrogels. (a)–(c) Scanning electron microscopy images depicting OGG/CMCS hydrogels with OGG concentrations of 1.0% (a), 1.5% (b), and 2.0% (c), respectively. Scale bar: 20 µm. (d)-(e) Frequency-dependent (d) and strain-dependent (e) rheology analysis of the hydrogels. (f) Compressive stress-strain profiles of the hydrogels. (g) Histogram of the maximum compressive stress of hydrogels. (h) Viscosity changes in hydrogels with increasing shear rates. (i) Injectability and extrudability of the hydrogel through a syringe. (j) Stretchability assessment of the hydrogel. (k) Variation of G’ and G’’ with time during continuous step strain measurement in 1% and 400% strain cycles. (l) Light transmittance of the hydrogels across wavelengths from 400 to 750 nm.
Notably, achieving high transparency in visible light is crucial for a corneal patch to avoid hindering vision during corneal repair. Thus, the light transmittance of OGG/CMCS hydrogels was evaluated on different wavelengths (Fig. 2(l)). All hydrogel samples with different formulations exhibited remarkable transparency, although the transmittance experienced a slight decrease with the increase in OGG concentration. The swelling and degradation characteristics of OGG/CMCS hydrogels were also evaluated. The OGG/CMCS-2% formulation demonstrated lower swelling capacity (Figure S4(a)) and slower degradation (Figure S4(b)) during incubation in PBS compared to both OGG/CMCS-1.5% and OGG/CMCS-1% hydrogels, suggesting a concentration-dependent behavior.
Furthermore, the OGG/CMCS hydrogels demonstrated effective tissue adhesive properties. The green-dye-containing hydrogels were in situ formed on porcine skin, displaying robust adherence under stretch, distortion, and bending (Fig. 3(a)). After immersing in water, the hydrogels could still adhere to porcine skin, even under distortion (Figure S5). This suggests a robust tissue-binding capacity of the hydrogel. Further comparisons of adhesiveness among different formulations were conducted through wound closure and lap shear tests. The OGG/CMCS-2% hydrogel demonstrated significantly higher adhesion strength (33.5 ± 2.78 kPa) than both OGG/CMCS-1.5% (26.7 ± 2.25 kPa, p p 3(b)). Similarly, OGG/CMCS-2% hydrogel demonstrated significantly higher shear strength (7.1 ± 0.4 kPa) than both OGG/CMCS-1.5% hydrogel (5.6 ± 0.4 kPa, p p 3(c)), suggesting the adhesive properties exhibited a concentration-dependent behavior.
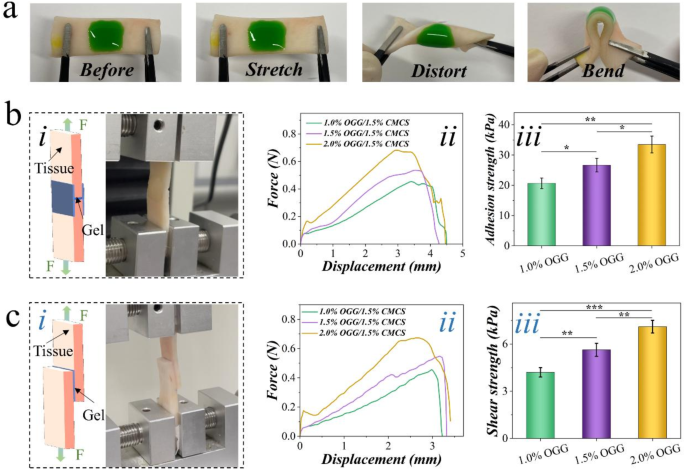
Adhesion properties. (a) Photographs of OGG/CMCS hydrogels adhered to porcine skin tissue. The hydrogel remains intact without detachment or cracking with the tissue, even during stretching, distorting, or bending. (b)-(c) Tissue adhesive properties of the hydrogels: (i) schematic representation and photography of the test; (ii) force-displacement curves; (iii) histogram of the adhesion (b) and shear strength (c) (*p p p
To assess biocompatibility, the liquid extracted from the OGG/CMCS hydrogel was utilized for culturing human corneal epithelial cells (HCECs) and keratocytes (HKs). Cells were cultured with hydrogel extracts (0, 5, 10, and 20 mg/mL) separately and cell viability was evaluated on days 1, 2, and 3 using the CCK-8 assay. On day 2, the viability of HCECs in extract liquid at all three concentrations was significantly higher than the control (normal medium), and the viability of HKs in extract liquid at 20 mg/mL was significantly higher than the control (Figs. 4(a) and 4(b), all p 20,21,22,23,24] (Figure S6).
To evaluate the quality of MSC-Exos, their concentration was quantified using nanoparticle tracking analysis (NTA), revealing an original concentration of 7.3 × 1010 particles/mL (Fig. 4(c)). Furthermore, the morphology of MSC-Exos was observed via transmission electron microscopy (TEM). The acquired Exos exhibited a characteristic saucer shape, featuring small vesicles with a central depression, in line with prior observations (Fig. 4(d)). Furthermore, the MSC-Exos expressed CD9 and CD63 exosomal markers (Fig. 4(e)). Following a 12-hour co-culture of MSC-Exos with HCECs, Exos effectively entered HCECs, indicating their easy endocytosis or internalization by HCECs (Fig. 4(f)). Additionally, the biological impact of MSC-Exos incorporated OGG/CMCS hydrogel on HCECs was investigated using a wound healing assay (Fig. 4(g)-4(h)). We detected a significant enhancement in scratch closure at 12 and 24 h with the application of MSC-Exos-loaded hydrogel compared to the control (p
The release profile of exosomes from the OGG/CMCS hydrogel was shown in Fig. 4(i), indicating a sustained and slower release of OGG/CMCS-2% hydrogel than OGG/CMCS-1.5% and OGG/CMCS-1% for 72 h after encapsulation of exosomes.
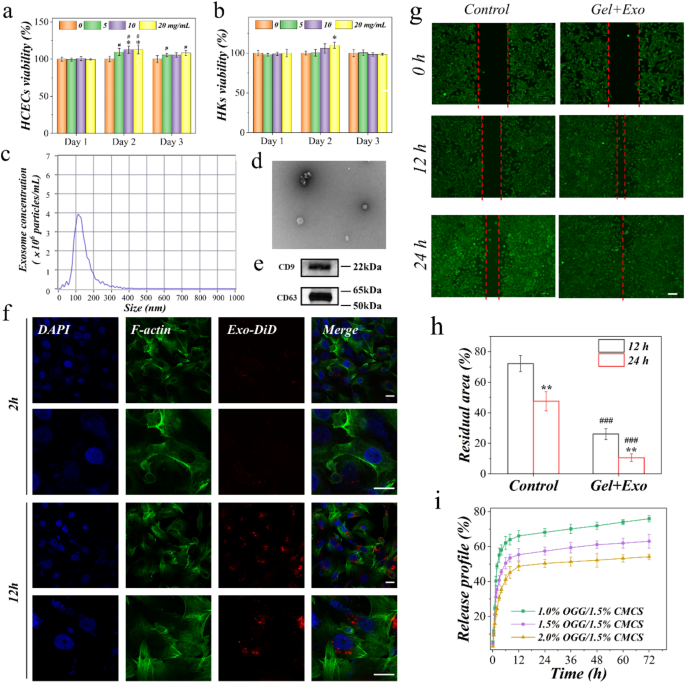
Biocompatibility of OGG/CMCS hydrogels and biological activity of mesenchymal stem cell exosomes (MSC-Exos) in the hydrogels. (a-b) Cell viability of human corneal epithelial cells (HCECs, a) and keratocytes (HKs, b) cultured with different concentrations of hydrogel patch extract following 1, 2, and 3 days (*p #p c) Size distribution profile of exosomes (following 1000-fold dilution). (d) Transmission electron microscopy of MSC-Exos. Scale bar: 200 nm. (e) Western blot analysis of the exosome surface markers. (f) Confocal microscopy of cellular uptake of DiD-labeled MSC-Exos by HCECs after culturing for 2 h and 12 h. Scale bar: 20 μm. (g) Scratch wound assay. Representative micrographs of HCECs treated with MSC-Exos-loaded OGG/CMCS hydrogel for 0, 12 h, and 24 h. Scale bar: 100 μm. (h) Quantitative evaluation of the scratch wound using ImageJ (**p ###p i) Release profile of exosomes from the OGG/CMCS hydrogel
After in vitro characterization and assessment of the OGG/CMCS hydrogels, we selected the hydrogel formulation with 2.0% OGG and 1.5% CMCS to assess its performance in vivo due to its superior mechanical strength, tissue adhesive properties, slower swelling rate, and controlled release of exosomes. We implanted the OGG/CMCS hydrogel subcutaneously in mice for 4 weeks to evaluate its in vivo biocompatibility. Histological examination showed that OGG/CMCS hydrogel implantation caused no lesions in the heart, liver, spleen, lung, or kidney in comparison to mice in the control group (Figure S7).
A rabbit corneal defect model was then used to evaluate the treatment effects. The amount of exosome encapsulation in the hydrogel is 8.75 × 109 particles/mL. The corneal defect was treated with the MSC-Exos-loaded OGG/CMCS hydrogel, pure hydrogel, and MSC-Exos respectively, while an untreated group was used as the control (Fig. 5(a)). After gelation, both hydrogels with and without MSC-Exos adhered effectively to the defected area, exhibiting smooth surfaces (Fig. 5(b)). The defected area was monitored in four groups at 1, 2, 4, and 8 weeks (Figs. 5(c)-5(e), Figure S8). Corneal transparency and the integrity of the corneal surface were assessed using diffuse illumination and a narrow slit beam. The corneal defect area remained smooth and transparent in eyes treated with MSC-Exos-loaded OGG/CMCS hydrogel during the 8 weeks post-surgery, while it exhibited mild opacity in the control and pure hydrogel groups at the 8th week (Figs. 5(c)-5(e)). Further, fluorescein staining was utilized to observe the corneal re-epithelialization. On the 7th day after surgery, eyes treated with MSC-Exos-loaded OGG/CMCS hydrogel exhibited minimal detection of green fluorescent staining in the epithelial defect area, contrasting with the relatively larger staining observed in the other groups (Fig. 5(c)), suggesting the novel hydrogel’s potential to enhance corneal epithelium healing. Anterior segment OCT was used to evaluate the process of hydrogel degradation and wound healing. The hydrogel displayed varying light reflectivity compared to the corneal stroma in OCT. The healing process occurred as the hydrogel gradually degraded and the healing was notably accelerated in the eyes treated with the MSC-Exos-loaded OGG/CMCS hydrogel compared to the other groups (Figs. 5(c)-5(e) and Figure S8). The hydrogel eventually degraded completely, leaving no permanent material in the cornea.
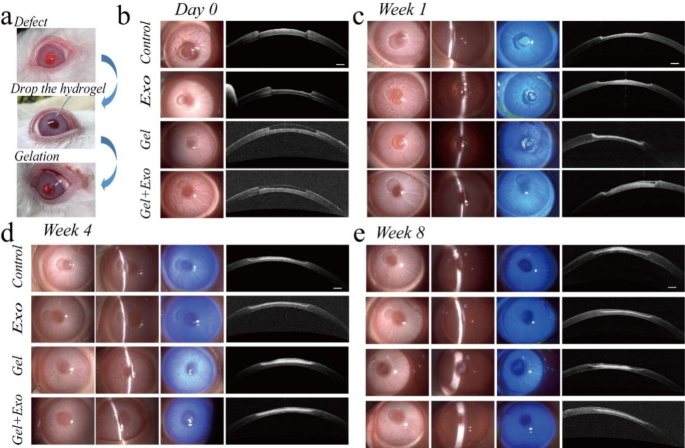
In vivo assessment of the MSC-Exos-loaded OGG/CMCS hydrogels in a rabbit corneal defect model. (a) Operation steps. (b)-(e) Representative slit lamp photos (diffuse illumination, narrow slit beam, and sodium fluorescein staining) and optical coherence tomography (OCT) images immediately (b), 1 (c), 4 (d), and 8 (e) weeks after surgery. Scale bar: 500 μm
Besides, histological examinations at 4 and 8 weeks revealed corneal repair in the defect area (Fig. 6(a)). In eyes treated with the MSC-Exos-loaded OGG/CMCS hydrogel, the thickness of epithelium and stroma in the defect area closely resembled that of the surrounding cornea, and the ratio of corneal stroma thickness to epithelial thickness was significantly greater than that in eyes without treatment and eyes treated with pure OGG/CMCS hydrogel on week 8 (Fig. 6(b)). Meanwhile, the recovery rate of overall corneal thickness on week 8 was highest in eyes treated with Exos-loaded hydrogel (Fig. 6(c)). In contrast, the control and pure hydrogel groups exhibited epithelial overgrowth, yet the stromal layer and the overall corneal thickness were significantly smaller compared to normal conditions. The precise arrangement of collagen fibrils in stroma is crucial for maintaining corneal clarity and optimal stromal hydration [1]. Masson trichrome staining illustrated that in the eyes treated with MSC-Exos-loaded OGG/CMCS hydrogel, the stroma maintained a well-arranged lamellar structure in contrast to the eyes treated with pure hydrogel (Fig. 6(a)). The cornea treated with Exos-loaded OGG/CMCS hydrogel exhibited higher coverage of collagen fiber (blue) in the corneal stroma compared to the Exos and pure hydrogel groups (Fig. 6(d)), indicating its potential to promote collagen fiber regeneration, while significant fibrosis (red) was observed in the repaired corneal stroma of Exos and pure hydrogel groups. It may contribute to better corneal transparency following healing with the assistance of the exosomes-loaded hydrogel.
Additionally, immunofluorescence staining for alpha-smooth muscle actin (α-SMA) and CD45 was utilized to evaluate the local fibrosis and inflammatory response in week 4. (Figs. 6(e) and 6(f)). The untreated wounded cornea exhibited higher expression of α-SMA at the wound edge compared to the cornea treated with MSC-Exos-loaded OGG/CMCS hydrogels (Fig. 6(e)). Activated keratocytes near the wound surface likely underwent differentiation into α-SMA-positive myofibroblasts in the injured cornea [25]. Exosome-loaded hydrogels significantly reduced α-SMA expression, demonstrating their potential to prevent myofibroblast formation and corneal scarring. Leukocyte infiltration, as indicated by CD45 expression, was lower in the cornea defects treated with exosome-loaded OGG/CMCS hydrogels in contrast to the control and pure hydrogel groups, suggesting the ability of exosome-loaded hydrogels to mitigate inflammation during corneal regeneration (Fig. 6(f)). The findings indicated that the MSC-Exos-loaded OGG/CMCS hydrogel can provide an ideal environment to enhance corneal wound healing in vivo.
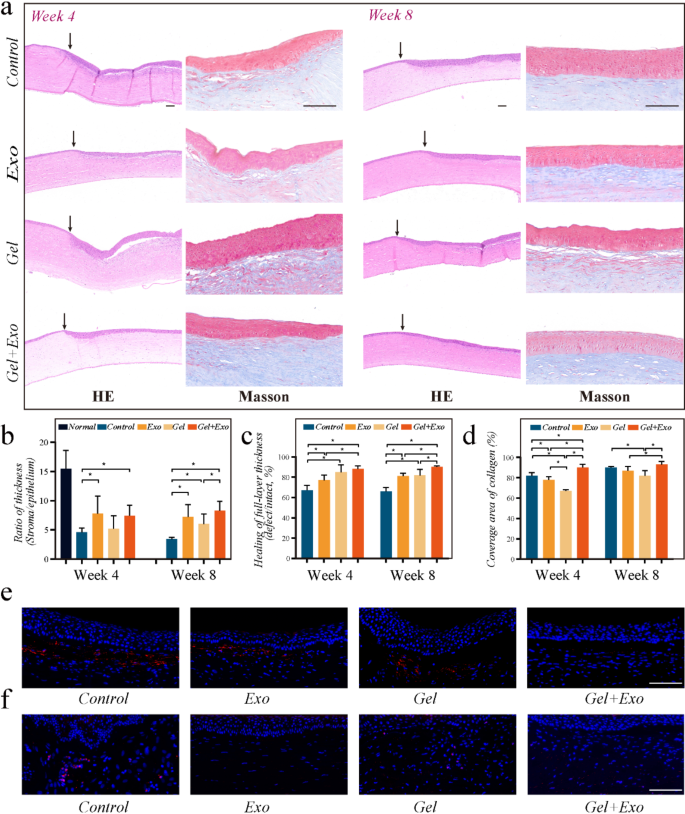
Histological evaluation of operated corneas. (a) HE and Masson staining on week 4 and 8 (arrows indicate the boundary of the corneal defect). Scale bar: 100 μm. (b) Ratio of corneal stroma thickness to epithelial thickness. (c) Recovery of corneal thickness. (d) Coverage area of collagen in corneal stroma. (e-f) Immunofluorescent staining of α-SMA (e) and CD45 (f) on week 4. Scale bar: 100 μm. (*p


