Synthesis and characterization of the OHADN fiber@Yoda1 hydrogel
The OHADN fiber@Yoda1 hydrogel was fabricated using a dual-crosslinking mechanism involving catechol-Fe³⁺ coordination and imine covalent bonds, as shown in Fig. 1a. The oriented fibers loaded with Yoda1 were prepared using the well-established electrospinning technique [19]. SEM analysis confirmed the uniform alignment of the fibers, as shown in Fig. 1b. This unidirectional orientation facilitates the precise cutting of the fibers into fragments. By controlling the thickness of the cryosectioning, we obtained fiber fragments of consistent size. The optimized segment length was approximately 20–50 μm, ensuring their uniform distribution within the hydrogel matrix without compromising injectability. As illustrated in the inset of Fig. 1c, a homogeneous translucent emulsion containing the fiber fragments was obtained. Considering that the tensile strength of the oriented fibers can reach up to 16 MPa, these fragments hold great potential for enhancing the mechanical properties of injectable hydrogels while maintaining their processability.
In biomaterial science, the native extracellular matrix component HA has been strategically repurposed as a multifunctional hydrogel precursor, particularly advantageous for minimally invasive delivery through injectable formulations due to its inherent shear-thinning behavior [30]. After oxidation, HA can be functionalized with aldehyde groups on its molecular chains, thereby offering enhanced possibilities for the construction of its hydrogels. OHA-based hydrogels have gained significant attention as filling materials for irregular bone defects due to their exceptional spatial adaptability [21, 22]. The distinctive linear macromolecular architecture of OHA contributes to hydrogel moisture retention and elastic properties. In this study, hydrogel formation was confirmed through vial inversion tests, with representative results shown in Fig. 1d.
The microstructure and elemental composition of the hydrogel were characterized using SEM and EDS. As shown in Fig. 1e, the OHADN fiber@Yoda1 hydrogel displayed a well-defined porous structure with a relatively uniform pore size distribution. This architectural feature is particularly beneficial for facilitating cell infiltration and nutrient transport, both of which are critical requirements for successful tissue engineering applications. Especially, interconnected fiber fragments were observed bridging the hydrogel walls, which is particularly significant for maintaining structural integrity during degradation. These residual fibers play a crucial role in preserving mechanotransduction signaling by sustaining cellular traction forces, thereby creating a favorable mechanical microenvironment that supports extended osteogenic differentiation processes. The EDS elemental mapping analysis (Fig. 1f and g) revealed that the OHADN fiber@Yoda1 hydrogel primarily consisted of carbon (C), nitrogen (N), oxygen (O), chlorine (Cl), and iron (Fe). The presence of these elements is consistent with the expected composition of the hydrogel system and suggests successful incorporation of the desired components.
OHA was synthesized through NaIO4-mediated oxidation of the proximal hydroxyl (-OH) group in HA. FTIR analysis (Fig. 1h) confirmed the aldehyde group formation in OHA, evidenced by a characteristic peak at 1736 cm⁻¹ corresponding to the -CHO group. Comparative analysis revealed enhanced OHADN peaks at 1530 cm⁻¹ (C––N stretching), 1450 cm⁻¹ (aromatic C–C stretching), and 1280 cm⁻¹ (C–N stretching) in the arrow-marked regions. These observations indicate successful dopamine grafting onto HA via Schiff base formation between the aromatic amine (-NH2) and OHA’s aldehyde group, consistent with reported mechanisms [29].
The matrix stiffness of hydrogels is a critical factor influencing cytoskeleton organization, cell migration, and differentiation [31, 32]. To investigate whether the incorporation of cross-linked fiber fragments could enhance the mechanical properties of OHADN hydrogels, we conducted rheological experiments to evaluate the storage modulus (G’) and loss modulus (G”). It is well-established that a higher G’ value compared to G” indicates the successful formation of a stable hydrogel network [29]. As shown in Fig. 1i, both the OHADN hydrogels and the OHADN fiber hydrogels exhibited G’ values that were consistently higher than their corresponding G” values across the frequency range of 10–100 rad s−1, confirming the formation of stable hydrogel networks. Furthermore, the G’ values of the OHADN fiber hydrogels were significantly higher than those of the OHADN hydrogels, demonstrating that the incorporation of cross-linked fiber fragments effectively enhanced the mechanical strength of the hydrogel. The elevated G’ values in the OHADN fiber hydrogels indicate greater resistance to deformation under applied stress, a property that is essential for maintaining structural integrity in dynamic physiological environments. This improved mechanical robustness not only ensures the hydrogel’s stability during cellular activities but also better replicates the mechanical properties of native tissues, which is critical for supporting cell function and bone regeneration [7, 33].
The adhesive properties of implanted materials are crucial for ensuring the retention of filling materials in irregular jaw defects. To evaluate the injectability and adhesion of OHADN fiber hydrogels, we analyzed their viscosity-shear rate curves using a rheometer. As shown in Fig. 1j, the viscosity of the OHADN fiber hydrogels decreased with increasing shear rate, demonstrating their shear-thinning behavior and confirming their injectability. This property allows the hydrogel to be easily extruded through a syringe while maintaining its structural integrity, as evidenced by its ability to remain in a gel state post-injection (Fig. S1a). Furthermore, Fig. S1b illustrates that the OHADN fiber hydrogel exhibited strong tissue adhesion, a critical feature for effective defect filling and retention. It is worth mentioning that the adhesiveness of the OHADN fiber hydrogel can be attributed to its unique composition and crosslinking mechanism. The OHA molecular chains, rich in aldehyde and catechol groups, in combination with the coordination of Fe³⁺ ions and the covalent action of imine bonds, not only form a stable hydrogel network structure but also endow it with excellent interfacial adhesive properties. This makes the hydrogel system an ideal material for repairing irregular mandibular defects.
When the hydrogel is injected to fill irregular bone defects, self-healing ability is essential to ensure the formation of a continuous barrier that protects against microbial invasion [34, 35]. As demonstrated in Fig. S1c, the OHADN fiber hydrogel exhibited remarkable macroscopic self-healing properties. When cut, the hydrogel rapidly healed without external intervention, and the healed hydrogel showed no visible cracks and could be lifted intact. This is the result of the combined action of dual dynamic chemical bonds. The self-healing capability not only ensures structural integrity but also enhances the hydrogel’s ability to maintain a protective barrier in dynamic physiological environments, making it highly suitable for applications in bone defect regeneration [36].
To validate the crosslinking effect of fiber fragments in injectable hydrogels, we systematically evaluated the degradation kinetics of OHADN fiber hydrogel. As depicted in Fig. 1k, OHADN fiber hydrogel and OHADN hydrogel exhibited comparable degradation rates during the initial 3-day period. However, a distinct divergence emerged from day 5 onward, with the OHADN fiber hydrogel demonstrating a slower degradation profile. By day 14, quantitative analysis revealed a 16% reduction in degradation rate of the OHADN fiber hydrogel compared to OHADN hydrogel (p 0.05). The result indicates that the covalent cross-linking network formed by the fiber fragments within the hydrogel system effectively retards the degradation kinetics of the hydrogel through a double-network reinforcement structure. Moreover, this observation aligns with established findings that cell-mediated hydrogel degradation facilitates cellular spreading and generates mechanical tension conducive to osteogenesis [7]. Following partial hydrogel degradation, the persistence of slower-degrading fiber fragments establishes anchorage platforms supporting cell migration, microenvironments enhancing cellular proliferation and topographic guidance for collagen matrix organization [26].
Enzymatic degradation experiment was conducted to analyze the release profile as shown in Fig. 1l. During hydrogel degradation (1–12 days), Yoda1 exhibited a release rate slope of 5.66%, which decreased to 0.53% post-hydrogel degradation, indicating that the drug-loaded fibrous fragments, when incorporated into the hydrogel matrix, exhibit an excellent sustained-release effect. Previous studies demonstrate electrospun fiber degradation durations up to 60 days [19]. The hydrogel phase initially promotes stem cell migration and spreading through rapid stress relaxation, while the Yoda1-loaded fiber network subsequently sustains mechanical tension and stabilizes PIEZO1 channels via sustained Yoda1 release to enhance osteogenic differentiation. Research confirms Yoda1, a low-molecular-weight compound, selectively activates PIEZO1 by stabilizing its open conformation and reducing mechanical activation thresholds [37]. Single-channel kinetic analysis reveals Yoda1-induced conformational changes: long-closed state occupancy decreased from 89.6 to 74.6%, long-open state increased from 7.3 to 22.5%, with slowed inactivation kinetics and accelerated recovery rates, ultimately achieving 2–3 times higher open probability [18]. These physicochemical properties collectively emphasize the imperative for systematic cellular investigations to validate the therapeutic potential of this hydrogel scaffold system in guiding stem cell fate.
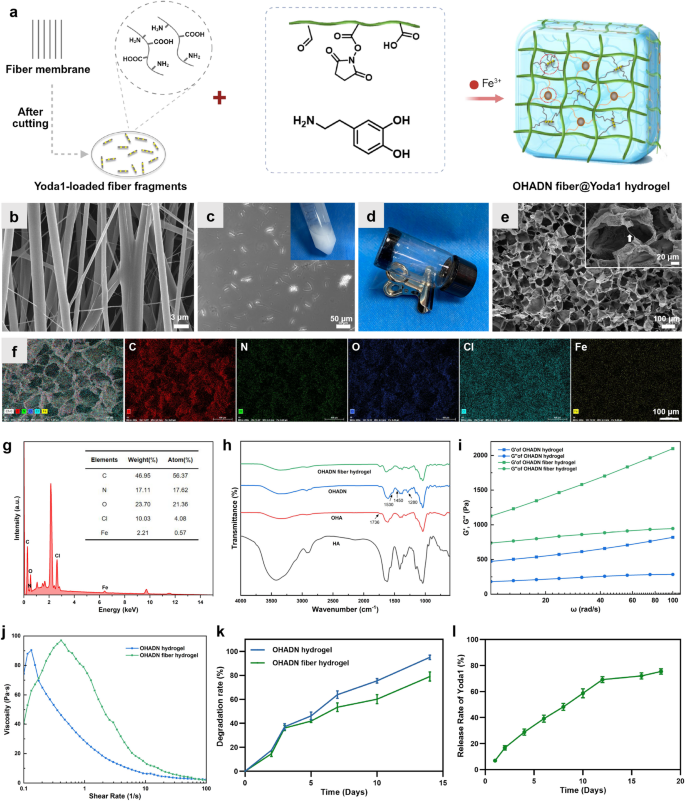
Synthesis and characterization of the OHADN fiber@Yoda1 hydrogel. (a) Schematic representation for fabricating the OHADN fiber@Yoda1 hydrogel. (b) SEM image of aligned fiber fabricating by electrospinning technique. (c) The image of fiber fragments, which the insets was PBS containing fiber fragments. (d) Vial inversion tests. (e) SEM image of OHADN fiber@Yoda1 hydrogel. The white arrows denote the fiber fragments (insets). (f and g) EDS elemental analysis of OHADN fiber@Yoda1 hydrogel. (h) FTIR spectra. (i) The storage modulus (G’) and loss modulus (G’’). (j) The viscosity-shear rate curves. (k) Degradation experiment. (l) The Yoda1 release curve
Effect of OHADN fiber@Yoda1 hydrogel on immune microenvironment
Macrophages, as versatile cellular components of the innate immune system, possess the remarkable capacity to dynamically respond to microenvironmental alterations and undergo polarization into diverse functional phenotypes, playing a pivotal role in tissue repair processes [38]. Mechanical stimuli, particularly matrix stiffness, have been demonstrated to promote the pro-inflammatory activation of macrophages, thereby facilitating the secretion of wound-healing cytokines that contribute to tissue regeneration [39]. However, excessive matrix stiffness may trigger severe foreign body responses and lead to fibrous capsule formation [40]. Consequently, it is imperative to systematically evaluate the inflammatory responses elicited by biomaterials at the cellular level.
Emerging evidence has demonstrated that PIEZO1 channel-mediated calcium influx plays a regulatory role in macrophage inflammatory responses and healing processes [40]. To investigate this mechanotransduction pathway, we employed calcium-sensitive fluorescent probes to quantify intracellular calcium levels in macrophages cultured on OHADN fiber@Yoda1 hydrogel group. As illustrated in Fig. 2a and b, both immunofluorescence imaging and microplate reader analyses consistently revealed a moderate elevation in intracellular calcium concentration in macrophages cultured on OHADN fiber@Yoda1 hydrogel compared to control group.
To further characterize the macrophage polarization state, we performed comprehensive analysis of inflammatory cytokine profiles at both transcriptional and secretory levels. RT-qPCR analysis (Fig. 2c) demonstrated statistically significant upregulation of anti-inflammatory genes ARG1 (P IL-10 (P IL-6 and TNF-α relative to control. Immunofluorescence results (Fig. 2d) revealed higher intensity of both ARG1 and iNOS in the OHADN fiber@Yoda1 hydrogel group compared to the control group; however, the iNOS/ARG1 ratio was significantly lower, indicating that the hydrogel induces a reparative microenvironment. Additionally, parallel assessment of secreted cytokines revealed a distinct pattern: substantial enhancement of Interleukin-4 (IL-4) and IL-10 production accompanied by significant suppression of multiple pro-inflammatory mediators, particularly Interleukin-1 (IL-1) and IL-6, though with a modest increase in Granulocyte-macrophage Colony-stimulating Factor (GM-CSF) and Interleukin-9 (IL-9) levels (Fig. 2e).
These results suggest that OHADN fiber@Yoda1 hydrogel promotes a hybrid macrophage phenotype that combines regenerative anti-inflammatory properties with essential immune surveillance functions, potentially creating a balanced microenvironment conducive to tissue repair while maintaining necessary immune responsiveness, as shown in the schematic diagram in Fig. 2f. These experimental observations align with the mechanistic insights provided by Wang et al., whose work elucidated that PIEZO1-mediated mechanotransduction not only enhances macrophage phagocytic efficiency and apoptotic cell clearance capacity but also orchestrates a phenotypic shift from pro-inflammatory to anti-inflammatory states through precise regulation of inflammatory marker expression [39].
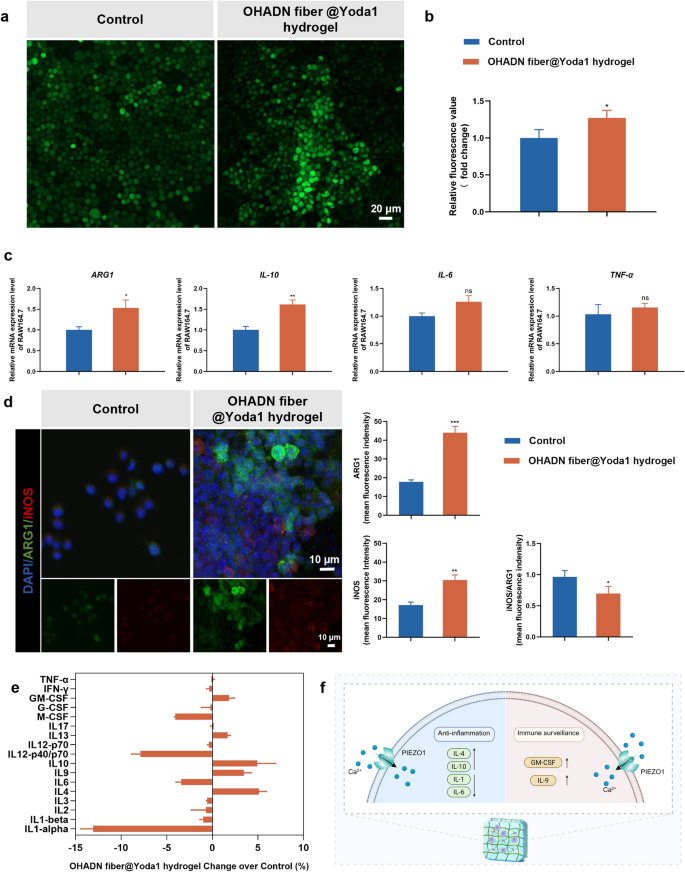
Effect of OHADN fiber@Yoda1 hydrogel on immune microenviroment. (a) Immunofluorescence imaging of intracellular calcium ion staining. (b) Relative fluorescence value of intracellular calcium ion detecting by microplate reader. (c) Quantitative RT-PCR analysis of inflammatory cytokine. (d) Immunofluorescence and semi-quantitative analysis, red for iNOS, green for ARG1, and blue for nuclei. (e) Inflammatory factors concentrations in culture medium supernatant. (f) The schematic diagram of hybrid macrophage phenotype promoting by OHADN fiber@Yoda1 hydrogel. (*P**P***P
Effect of OHADN fiber@Yoda1 hydrogel on cytocompatibility
The cytocompatibility of biomaterials is crucial for their clinical application [41]. To evaluate the biocompatibility of the hydrogels, BMSCs were seeded onto the hydrogel surfaces, and cell morphology was observed through cytoskeletal staining, while cell viability was assessed using the CCK-8 assay.
As shown in Fig. 3a, cells in all groups exhibited healthy morphological characteristics. Notably, cells cultured on the OHADN fiber@Yoda1 hydrogel displayed a larger spreading area compared to those on the OHADN hydrogel and OHADN fiber hydrogel, with no statistically significant difference observed relative to the control group. The CCK-8 results revealed no significant differences in OD values among the groups on day 1 (Fig. 3b). By day 3 and 5, the OD value of the OHADN fiber@Yoda1 hydrogel group was higher than that of the OHADN fiber hydrogel groups, with no significant difference compared to the control group. These results collectively demonstrate that the OHADN fiber@Yoda1 hydrogel exhibits excellent cytocompatibility.
The spreading area of cells on biomaterial surfaces is closely linked to the establishment and maintenance of cellular tensional homeostasis, a key factor regulating cell behavior and function [10]. When cells adhere to a substrate, they generate cytoskeletal tension through the formation of focal adhesions and actin stress fibers, which are essential for maintaining mechanical equilibrium within the cell [42]. The extent of cell spreading is directly influenced by the mechanical properties and surface characteristics of the biomaterial, such as stiffness, topography, and ligand density [31, 43]. These factors collectively determine the ability of cells to sense and respond to their microenvironment, a process known as mechanotransduction [44].
In the context of the OHADN fiber@Yoda1 hydrogel, the observed increase in cell spreading area suggests that this material provides an ideal mechanical microenvironment for cells to establish tensional homeostasis. The hydrogel’s composition and structural properties likely promote the formation of stable focal adhesions and actin stress fibers, enabling cells to generate and sustain the necessary cytoskeletal tension. This, in turn, supports cellular functions such as proliferation, differentiation, and migration, while enhancing the overall biocompatibility of the material.
Furthermore, the role of mechanosensitive ion channels, particularly PIEZO1, cannot be overlooked in this process. PIEZO1 channels are directly gated by membrane tension and play a crucial role in cytoskeletal reorganization and tissue homeostasis, acting as key sensors of mechanical equilibrium [15]. The low-molecular-weight compound Yoda1, which selectively targets PIEZO1 channels, enhances their sensitivity to mechanical stimuli [37]. In the OHADN fiber@Yoda1 hydrogel, the incorporation of Yoda1 may further amplify the mechanotransduction signals, promoting a more robust cellular response to the mechanical microenvironment provided by the hydrogel.
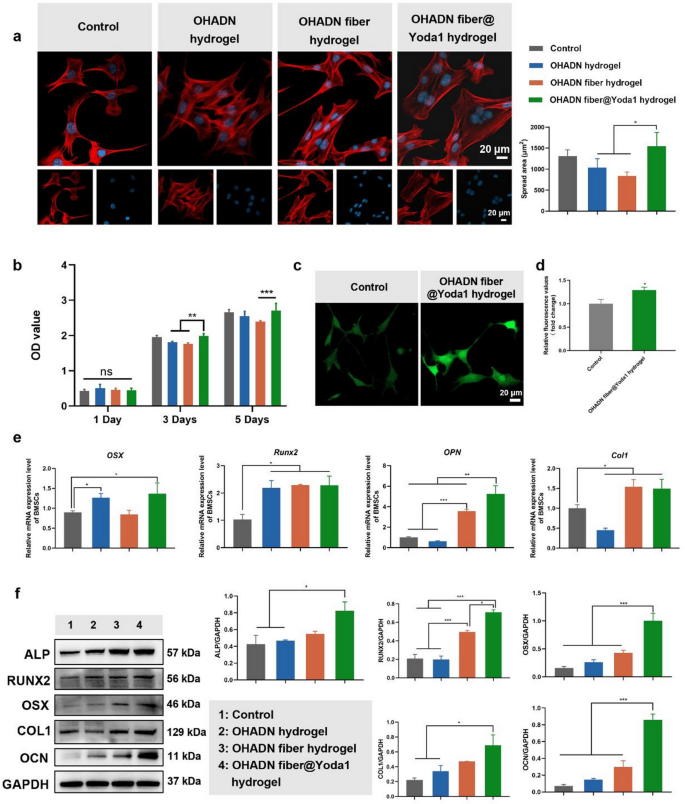
OHADN fiber@Yoda1 hydrogel possesses excellent cytocompatibility and osteogenic potential. (a) Cytoskeletal staining and cell-spread area analysis, blue for nuclei and red for F-actin. (b) The cytotoxicity of each group of materials was assessed by the CCK-8 assay. (c) Immunofluorescence imaging of intracellular calcium ion staining. (d) Relative fluorescence value of intracellular calcium ion detecting by microplate reader. (e) Quantitative RT-PCR analysis of osteogenic genes. (f) Western blot analysis of osteogenic proteins. (*P**P***P
OHADN fiber@Yoda1 hydrogel possesses excellent osteogenic potential
After confirming satisfactory cytocompatibility, BMSCs were seeded onto the surfaces of various materials and subjected to osteogenic induction for 7 days. Intracellular calcium levels were determined by staining with the fluo-4 probe as shown in Fig. 3c and d. The expression of osteogenic genes and proteins was evaluated using RT-qPCR, western blot and immunofluorescence staining, as shown in Fig. 3e and f and S2.
Intracellular calcium ion staining revealed that the OHADN fiber@Yoda1 hydrogel group exhibited a significantly higher fluorescence intensity compared to the control group, suggesting enhanced calcium influx mediated by this hydrogel. This phenomenon is presumably attributed to the activation of the mechanosensitive PIEZO1 ion channel, which is specifically potentiated by the Yoda1 agonist incorporated in the hydrogel system. As a prototypical mechanosensitive ion channel, PIEZO1 coordinates mechanochemical transduction by detecting plasma membrane tension variations [18]. This structural sensor initiates calcium ion influx through its characteristic trimeric propeller configuration, subsequently triggering mechanotransduction cascades involving Hippo pathway effectors YAP/TAZ+ [14]. The nuclear translocation of these transcriptional co-activators fundamentally governs the osteodifferentiation commitment of mesenchymal stem cells [17, 45, 46].
RT-qPCR results revealed that the OHADN fiber@Yoda1 hydrogel group exhibited increased expression of osteogenic genes, including OSX, Runx2, OPN, and Col1, compared to the control group. However, the differences in osteogenic gene expression levels among the material groups were inconsistent. Specifically, Runx2 expression was higher in all material groups compared to the control, with no significant differences observed between the material groups. The expression of OSX was significantly elevated in the OHADN hydrogel and OHADN fiber@Yoda1 hydrogel groups compared to the control, while the OHADN fiber hydrogel group showed no significant difference relative to the control. Western blot results further supported these findings, showing that the OHADN fiber@Yoda1 hydrogel group exhibited higher protein levels of ALP, RUNX2, OSX, COL1, and OCN compared to the control group, consistent with the RT-qPCR data. Furthermore, immunofluorescence staining demonstrated that the expression of RUNX2 in the OHADN fiber@Yoda1 hydrogel group was significantly higher than in the other three groups (Fig. S2). These results collectively indicate that the OHADN fiber@Yoda1 hydrogel possesses excellent osteogenic potential, effectively promoting osteoblast differentiation and maturation.
The observed upregulation of osteogenic genes and proteins in the OHADN fiber@Yoda1 hydrogel group highlights its superior ability to support osteogenic differentiation. RUNX2, a master regulator of early osteogenesis [47], was consistently elevated across all material groups, suggesting that changes in extracellular matrix stiffness universally influence RUNX2 expression. However, the differential expression of OSX, a downstream transcription factor critical for osteoblast maturation [47], indicates that not all materials can sustain the progression of osteogenic differentiation. The significant upregulation of OSX in the OHADN fiber@Yoda1 hydrogel group suggests that this material provides a conducive microenvironment for advanced osteogenic maturation.
The role of mechanotransduction in osteogenesis is further underscored by the involvement of PIEZO1 channels, which are known to mediate mechanical signals during bone development [13, 14]. Activation of PIEZO1 channels triggers cytoskeletal reorganization, which is essential for maintaining cellular tensional homeostasis and promoting bone regeneration. Our previous studies demonstrated that low-concentration Yoda1, a PIEZO1 activator, upregulates cytoskeleton-related genes and enhances osteogenic protein expression [19]. This aligns with the current findings, where the OHADN fiber@Yoda1 hydrogel, likely through PIEZO1-mediated mechanotransduction, promotes a robust osteogenic response.
The OHADN fiber@Yoda1 hydrogel not only enhances early osteogenic markers like RUNX2 but also supports the expression of late-stage markers such as OSX and OCN, indicating its potential to drive complete osteogenic differentiation. The integration of PIEZO1-mediated mechanotransduction further amplifies its osteoinductive properties, making it a promising candidate for bone tissue engineering applications.
OHADN fiber@Yoda1 hydrogel promotes bone regeneration via PIEZO1 – ITGα5 axis
To elucidate the molecular mechanisms underlying OHADN fiber@Yoda1 hydrogel-enhanced osteogenic differentiation of BMSCs, we conducted RNA sequencing analysis. High-throughput transcriptome sequencing was performed using the DNBSEQ platform, yielding an average of 6.44 GB clean data per sample across control and OHADN fiber@Yoda1 hydrogel groups. The alignment rates reached 98.96% for reference genomes and 80.38% for annotated gene sets, with 16,671 genes detected in total.
To assess the inter-sample correlations in gene expression, Pearson correlation coefficients of all gene expression levels between every two samples were calculated. These coefficients were then visualized in a heatmap (Fig. 4a), with the majority of correlation coefficients falling within acceptable limits. Venn diagram analysis identified 761 and 698 unique differentially expressed genes (DEGs) in control and OHADN fiber@Yoda1 hydrogel groups, respectively (Fig. 4b). Volcano plot analysis revealed 2,226 upregulated and 2,343 downregulated genes with statistical significance (Fig. 4c). Hierarchical clustering demonstrated distinct separation of expression patterns between groups. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of DEGs highlighted significant enrichment in focal adhesion, regulation of actin cytoskeleton, and stem cell pluripotency-related signaling pathways in the hydrogel group (Fig. 4d). Gene Set Enrichment Analysis (GSEA) further confirmed substantial enrichment of the focal adhesion pathway (NES = 1.60, P 4e), suggesting its potential involvement in osteogenic differentiation.
Notably, the OHADN fiber@Yoda1 hydrogel group exhibited significant upregulation of key osteogenic regulators within the focal adhesion pathway—Itgα5, Tns1, Spp1 (encoding OPN), and Id3 (a BMP signaling effector). This coordinated gene activation profile (Fig. 4f) reveals a mechanotransduction cascade where Yoda1-induced PIEZO1 activation primes mechanical signaling through the ITGα5-TNS1 axis, subsequently amplifying mineralization drivers (Spp1/OPN) and activating BMP-responsive transcription factors (Id3) to potentiate bone formation. RT-qPCR analysis demonstrated significantly upregulated mRNA expression levels of Itgα5, Tns1, Rhoa, Rock2, and Id3 in the OHADN fiber@Yoda1 hydrogel group compared to control groups (Fig. 5a). Western blot results in Fig. 5b and Fig. S3 further revealed that the OHADN fiber@Yoda1 hydrogel markedly enhanced protein expression of ITGα5, TNS1, RHOA, and ROCK2. These collective findings provide compelling evidence for the hydrogel’s dual functional mechanism, which concurrently activates mechanosensitive ion channels and orchestrates downstream osteogenic signaling pathways through spatiotemporal coordination. The schematic diagram in Fig. 5c delineates this complex working mechanism, highlighting the material’s capability to synchronize mechanical signal perception with biochemical pathway activation.
Transmembrane communication plays a critical role in responding to extracellular stimuli and maintaining intracellular homeostasis [48, 49]. The PIEZO1 channel serves as a key homeostatic sensor in shaping tissue homeostasis [15, 16]. After disrupting actin filaments with cytochalasin D, the current amplitude of PIEZO1 channels decreases, indicating that the cytoskeleton is responsible for transmitting mechanical stimuli upon PIEZO1 channel activation [15]. Atomic force microscopy colocalization experiments further reveal a strong interaction between PIEZO1 channels and the cytoskeleton [50]. The function of PIEZO1 channels extends beyond transient transduction of mechanical signals; they achieve long-term homeostatic balance in the tissue microenvironment by dynamically regulating cellular tension, metabolic activity, and intercellular communication.
On the other hand, ITGα5, a core receptor in cell-matrix interactions, transmits mechanical signals through focal adhesion complexes, while tensin regulates cytoskeletal reorganization. These two components synergistically maintain tension homeostasis [51]. This suggests that PIEZO1 channels and the integrin system may exhibit cooperative regulatory mechanisms. As the primary mechanical sensor of the extracellular matrix, integrin signaling and the mechanical response of PIEZO1 channels are highly coupled in both spatial and temporal dimensions. Recent studies have revealed that PIEZO1 channels and integrin β1 on cardiac fibroblast surfaces mutually activate to form a positive feedback loop [52, 53]. Stiffer extracellular matrices upregulate PIEZO1 channel activity via integrin β1 [54], implying that integrins may indirectly modulate PIEZO1 channel activity by regulating cytoskeletal tension.
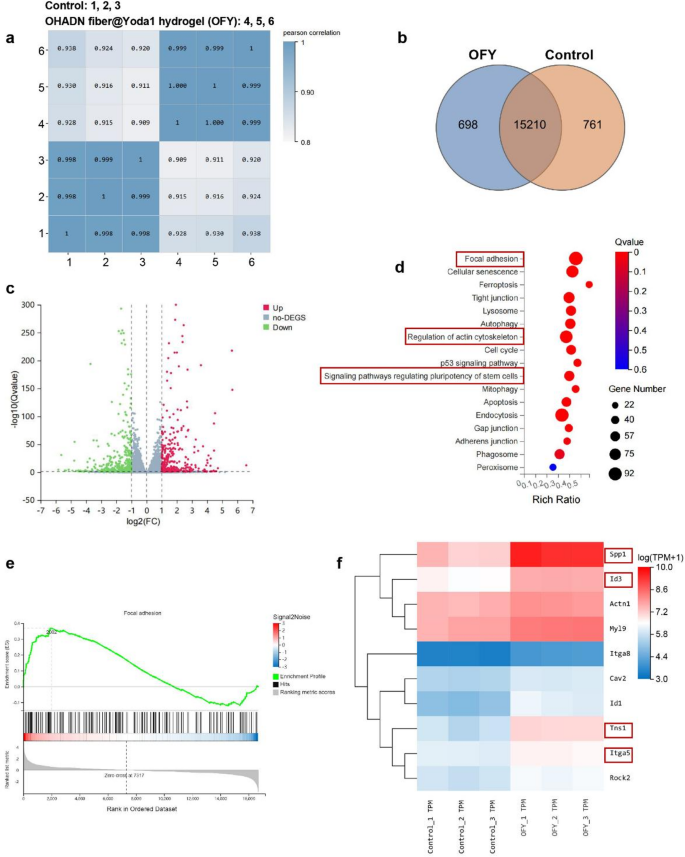
Transcriptome analysis of the OHADN fiber@Yoda1 hydrogel. (a) Pearson correlation coefficients between every two samples. (b) Venn diagram analysis. (c) Volcano plot analysis. (d) KEGG pathway enrichment analysis. (e) GSEA analysis. (f) Heatmap of the represented up-regulated genes
v
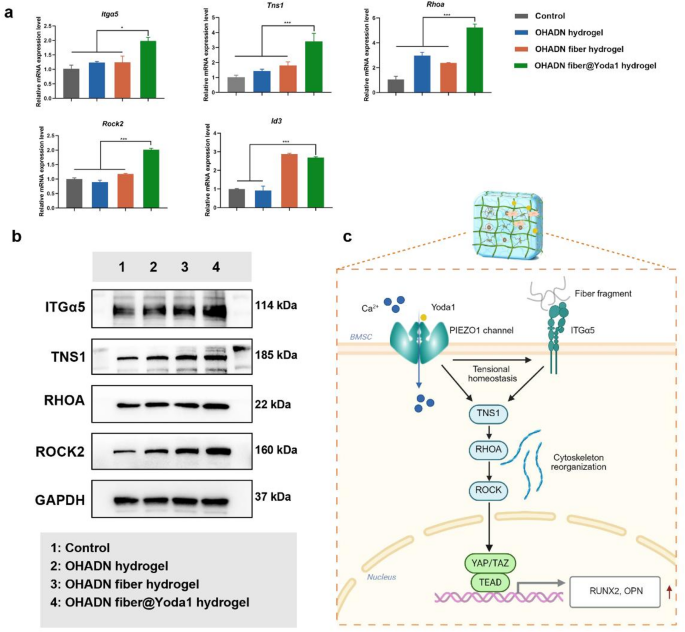
OHADN fiber@Yoda1 hydrogel promotes bone regeneration via PIEZO1 – ITGα5 axis. (a) Quantitative RT-PCR analysis of Itgα5, Tns1, Rhoa, Rock2, Id3. (b) Western blot assay of ITGα5, TNS1, RHOA, and ROCK2. (c) Schematic of OHADN fiber@Yoda1 hydrogel promoted bone regeneration through the PIEZO1 – ITGα5 axis. (*P**P***P
OHADN fiber@Yoda1 hydrogel promoted alveolar bone regeneration in vivo
To investigate the in vivo osteogenic performance of OHADN fiber@Yoda1 hydrogel, we established a rat alveolar bone defect model [55, 56] and implanted experimental materials into the bone defects (Fig. 6a). Bone regeneration efficacy was systematically evaluated using Micro-CT, H&E staining, Masson’s trichrome staining, and immunohistochemical analyses. Given the well-documented osteoinductive properties of hydroxyapatite, a material widely adopted in bone substitute applications, it was selected as the positive control [57, 58]. A blank control group without material implantation was included for baseline comparison.
Initial biocompatibility assessment through H&E staining of visceral organs (heart, liver, spleen, lungs, kidneys) revealed no discernible pathological alterations in any experimental group after 4-week implantation, confirming the absence of systemic toxicity and further validating the biosafety profile of OHADN fiber@Yoda1 hydrogel (Fig. S3).
Micro-CT analysis with three-dimensional reconstruction (Fig. 6b) demonstrated superior bone regeneration metrics in the OHADN fiber@Yoda1 hydrogel group. Specifically, this group exhibited a significantly higher bone volume fraction (BV/TV) compared to the blank control, OHADN hydrogel, and OHADN fiber hydrogel groups (P P > 0.05). A similar trend was observed in Tb.Th measurements, suggesting enhanced osteogenic capacity. Notably, the OHADN fiber@Yoda1 hydrogel group displayed significantly lower Tb.Sp than the blank control and OHADN hydrogel groups (P P > 0.05). The reduced Tb.Sp observed in the experimental group aligns with previous reports that interconnected fiber networks in composite hydrogels can guide osteoblast migration and enhance matrix mineralization. These findings collectively indicate superior quality of neoformed bone in the OHADN fiber@Yoda1 hydrogel group.
Consistent histological observations were obtained through H&E and Masson’s trichrome staining analyses (Fig. 6c). Analysis of newly formed bone tissue within the demarcated regions (white dashed lines) demonstrated superior bone formation in the OHADN fiber@Yoda1 hydrogel group compared to other experimental cohorts. These findings validate the outstanding osteogenic potential and structural guidance capabilities of the OHADN fiber@Yoda1 hydrogel.
We further conducted immunohistochemical analysis to evaluate the expression levels of osteogenic proteins and signaling pathway components. As shown in Fig. 7a, the OHADN fiber@Yoda1 hydrogel group demonstrated significantly higher RUNX2 and OPN expression compared to control, OHADN hydrogel, and OHADN fiber hydrogel groups, while showing comparable levels to the Hydroxyapatite group. Figure 7b revealed that PIEZO1, ITGα5, and TNS1 expression in the OHADN fiber@Yoda1 hydrogel group markedly exceeded those in all other groups. Importantly, immunohistochemical co-staining analysis of OHADN fiber@Yoda1 hydrogel group demonstrated spatial co-localization of PIEZO1 and ITGα5 (Pearson correlation coefficient = 0.51, Fig. 7c), supporting their functional interaction in mechanotransduction.
The observed upregulation of PIEZO1 expression may be attributed to Yoda1-induced activation of PIEZO1 channels through conformational changes, subsequently triggering increased ITGα5 and TNS1 expression which in turn regulates PIEZO1 channel expression, suggesting a potential positive feedback loop. This finding indicates a possible synergistic regulatory mechanism between PIEZO1 channels and the integrin system, which is structurally underpinned by their co-localization at focal adhesions. This mechanism is further supported by evidence of mechanical coupling: PIEZO1 colocalizes with integrins, and stiff matrices enhance PIEZO1 activity via integrin β1-mediated cytoskeletal tension [50, 52,53,54]. Recent finding also showed that PIEZO1 physically localizes to focal adhesions to regulate integrin-FAK signaling assembly [59]. Specifically, PIEZO1 knockdown impairs focal adhesion formation and abrogates integrin-FAK pathway activation, confirming its essential role in mechanosensitive adhesion complexes.
Leveraging these cooperative mechanisms, the OHADN fiber@Yoda1 hydrogel achieves spatiotemporal control of mechanosignaling through its biphasic architecture: the initial hydrogel phase facilitates stem cell migration via rapid stress relaxation, while the fiber network sustains mechanical tension and provides controlled Yoda1 release to stabilize the PIEZO1-ITGα5 signaling hub. Yoda1 sensitizes PIEZO1, enabling Ca²⁺ influx comparable to rigid substrates despite the hydrogel’s sub-rigid properties [18]. This calcium influx activates RHOA/ROCK-mediated cytoskeletal contraction [60], elevating ITGα5 expression and enhancing collagen binding for osteogenic differentiation [61]. Collectively, our findings demonstrate that the OHADN fiber@Yoda1 hydrogel effectively promotes alveolar bone regeneration through the PIEZO1-ITGα5 mechanosignaling axis. While our data suggest possible crosstalk through membrane deformation when these mechanosensors are in proximity, we acknowledge that fully elucidating the direct molecular interplay within the PIEZO1-ITGα5 axis remains a limitation requiring further mechanistic validation in ongoing studies.
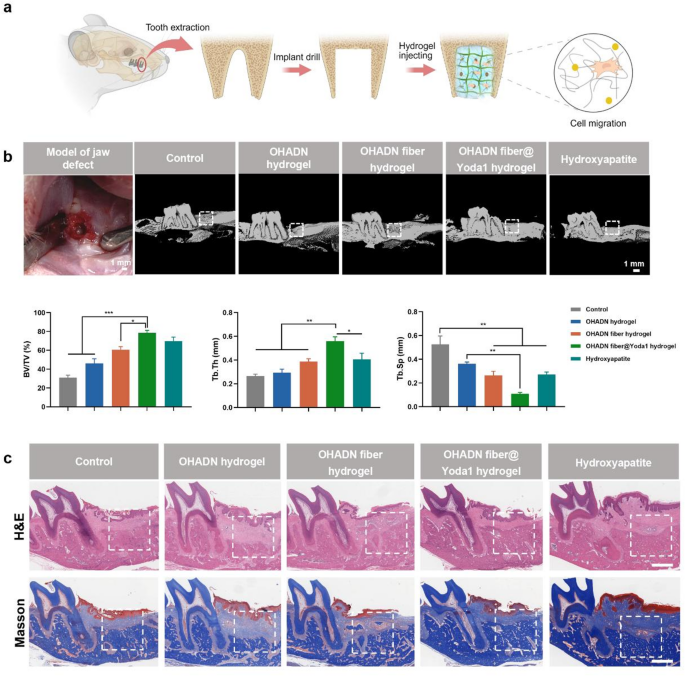
OHADN fiber@Yoda1 hydrogel promoted alveolar bone regeneration in vivo. (a) A schematic diagram of the animal experiment. (b) The 3D reconstruction of micro-CT and analysis of bone volume fraction (BV/TV), trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp). (c) Representative H&E and Masson’s trichrome staining images of various group at week 4. The white dashed box represents the defect location. Scale bars represent 1 mm. (**P***P
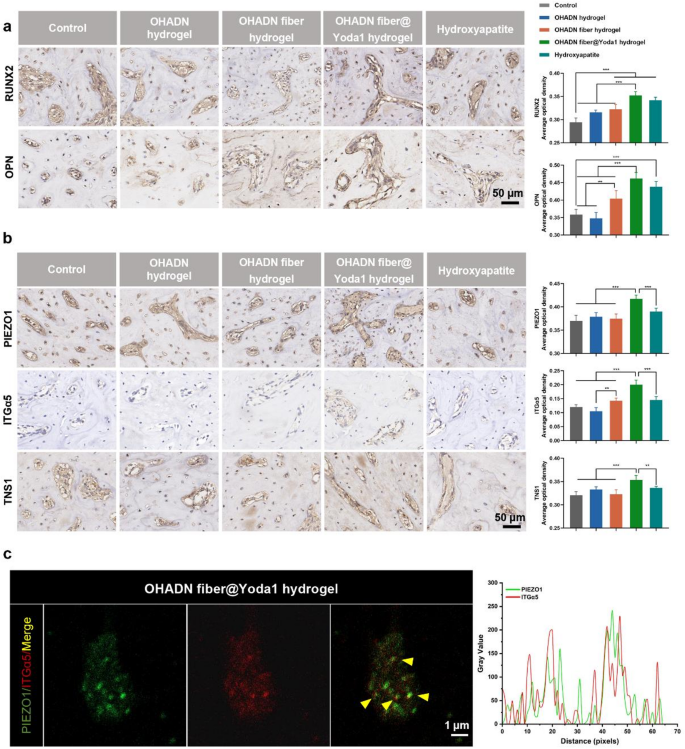
OHADN fiber@Yoda1 hydrogel promoted alveolar bone regeneration in vivo. (a) Immunohistochemical staining and quantification of average optical density for RUNX2 and OPN of the alveolar bone defect. (b) Immunohistochemical staining and quantification of average optical density for PIEZO1, ITGα5 and TNS1 of the alveolar bone defect. (c) Immunohistochemical co-staining of PIEZO1 (green) and ITGα5 (red). Yellow indicates their spatial co-localization. Right panel: Quantitative curve of the fluorescence assay analyzed by Image J software. (**P***P


