Preparation and characterization of the VMn-Gd@GOx-EVs
Here, virus-mimic hollow mesoporous Mn-Gd hybrid nanocarriers (VMn-Gd) were synthesized utilizing a conventional hard template strategy (Fig. 1A). Initially, the hard template, virus-mimic mesoporous SiO2 nanotemplate (VSN), was produced via a mono-micelle epitaxial growth method in a biphasic reaction system (water/cyclohexane) as previously reported. In this process, CTAB and TEA served as surfactant and reductant, respectively, and were dissolved in deionized water. TEOS, the silica precursor, was added to cyclohexane in a 1:4 volume ratio and carefully dripped onto the aqueous phase. After 72 h, VSN was obtained, exhibiting unique morphology and excellent mono-dispersity with a very uniform size distribution (~ 100 nm). TEM and SEM images revealed that numerous spinous nanotubes (approximately 5–10 nm) were radially assembled on the spherical surface of VSN (Fig. 1B, F). Subsequently, Mn-Gd precursors, Mn(NO3)2•6H2O and Gd(NO3)3•6H2O in a 3:2 molar ratio, were used to form a MnO2-Gd2O3 hybrid shell around VSN (VSN@Mn-Gd) under hydrothermal conditions with weak alkali methylamine (Fig. 1C). The spinous nanotubes facilitated in situ nucleation of the Mn-Gd precursor hydrolysis. VSN was then removed using 0.1 M NaOH, resulting in distinctive hollow Mn-Gd bimetallic oxide nanocarriers (VMn-Gd) with a uniform diameter of approximately 110 nm (Fig. 1D). Figure 1G shows that VMn-Gd retains the virus-mimic morphology of VSN, including the nanospikes on the spherical surface. Subsequently, GOx were encapsulated in VMn-Gd and the loading amount of GOx was determined using a Bicinchoninic Acid (BCA) Protein Assay kit. In VMn-Gd@GOx, the enzyme loading was found to be ∼0.21 ± 0.13 mg-GOx/mg-VMn-Gd with the loading efficiency as 17.35%. Finally, VMn-Gd@GOx was coated with EV membranes (VMn-Gd@GOx-EVs) using gentle sonication at 4 °C followed by filter extrusion. TEM confirmed the presence of the outer membrane layer, with the size slightly increasing by 10 nm (Fig. 1E). SEM images indicated that the virus-like nanostructure of VMn-Gd@GOx became blurred in VMn-Gd@GOx-EVs (Fig. 1H). These findings confirmed the successful encapsulation EVs on the virus-like nanoagents. High-angle annular dark field scanning electron microscopy (HADDF-SEM) was used to analyze elemental distribution, revealing homogeneously dispersed Si, O, Mn, and Gd elements in the nanocarriers (Fig. 1I), with further confirmation from elemental mapping analysis in Fig. S1. Encouraged by these results, we evaluated the tumor microenvironment-responsive capability of our nanoplatform. TEM images recorded after incubating Mn-Gd@GOx in PBS (1X) at pH 7.4 or 6.5 for different times (Fig. 1J) showed no significant morphological changes at pH 7.4 even after 36 h, indicating stability under neutral conditions. However, VMn-Gd@GOx displayed a clear time-dependent degradation at pH 6.5, with half of the nanoshells decomposing at 12 h and complete collapse after 36 h. Particularly, compared to traditional Gd-based nanosensitizers used for radiotherapy [44, 45], our VMn-Gd@GOx-EVs degrade under mildly acidic tumor microenvironment conditions, releasing small particles around 10 nm after 36 h (Fig. 1J), which further promotes tumor penetration. Excitingly, after incubation for 48 h at pH 6.5, the release efficiency of GOx was more than 7 times higher than that at pH 7.4 (Fig. S2). These results demonstrate the nanoplatform’s sensitivity to acidic tumor microenvironments, making it suitable for precise cargo delivery, early diagnosis and the subsequent synergistic therapy for tumors.
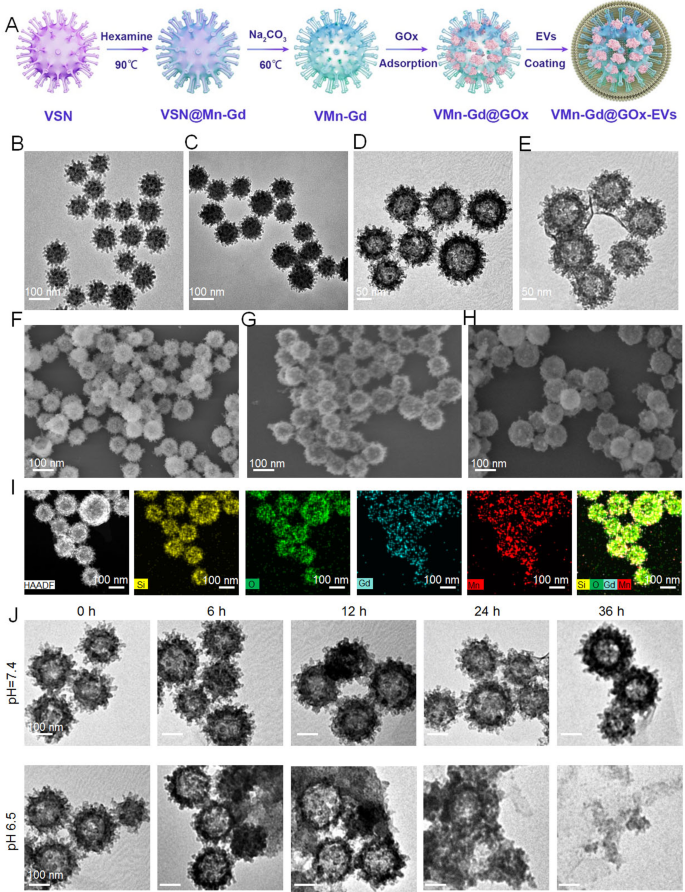
(A) A schematic representation of the fabrication process for VMn-Gd@GOx-EVs. TEM images showcasing (B) VSN, (C) VSN@Mn-Gd, (D) VMn-Gd, and (E) VMn-Gd@GOx-EVs. SEM images displaying (F) VSN, (G) VMn-Gd, and (H) VMn-Gd@GOx-EVs. (I) HADDF images illustrating the uniform elemental distribution of Si, O, Mn, and Gd in VMn-Gd@GOx. (J) TEM images of VMn-Gd@GOx after being immersed in buffers with different pH levels (6.5, 7.4) for various durations
MRI capability, GSH consumption, GOx depletion and ROS generation in bulk solution
Owing to Mn and Gd ions can be triggered to release in tumor acid microenvironment, then we assessed the performance of T1-weighted contrast-enhanced MRI, VMn-Gd@GOx-EVs and gadopentetic acid (Gd-DTPA) with different Gd3+ concentrations were imaged using a 9.4 T MRI system. As shown in Fig. 2A, Mn-Gd@GOx-EVs showed more significant T1 MRI signals than Gd-DTPA, which was the most common MRI contrast agent at the clinic. The r1 relaxivity of Mn-Gd@GOx-EVs was 12.90 mM−1 s−1, which was ~ 2-fold higher than that of Gd-DTPA (6.368 mM−1 s−1) (Fig. 2B). Furthermore, the brightness of T1-weighted MRI images increased with Gd3+ concentration, suggesting that Gd3+ in our naonsystem could effectively enhance the MRI signals for accurate tumor delineating. Subsequently, to evaluate the radio-enhancement effect of VMn-Gd@GOx, an aminophenyl fluorescein (APF) probe was used to capture •OH (Fig. 2C). As shown in Fig. 2D, VMn-Gd and VMn-Gd@GOx generated equivalent quantities of •OH that prominently higher than PBS upon X-ray irradiation (8 Gy) with ~ 2-fold relative enhancements. In addition, the production of •OH was related to the radiation dose, demonstrating that X-rays were the key factor for inducing •OH generation. Subsequently, the enzyme activity of VMn-Gd@GOx (Fig. S3) was firstly examined under various glucose conditions. Results showed that a high glucose level induced more H2O2 generation within 5 h incubation reaching the plateau. Notably, the generated H2O2 quickly reached an equilibrium within only 0.6 h, demonstrating that the VMn-Gd@GOx possessed strong and rapid glucose decomposition capability for H2O2 generation (Fig. 2E). The generate pH variation during glucose consumption also showed a similar tendency, where a high glucose level led to a greater pH decrease (from 7.4 to 3.5) (Fig. 2F). The pH decrease caused by the production of gluconic acid could trigger the degradation of VMn-Gd@GOx, which was demonstrated by the TEM images (Fig. 1J). Besides, this induced lower pH condition (4 ~ 5) can be facilitate for the subsequent Fenton-like reaction of Mn ions. Collectively, either pH decline or the H2O2 increase can significantly mediating the biodegradation of Mn-Gd@GOx and the subsequent •OH generation, making the as-prepared VMn-Gd@GOx an excellent nanocarrier for tumor acidity-responsive drug delivery and oxidative therapy. Hence, the produced •OH through a Fenton-like reaction was latterly appraised in diverse of pH values. To evaluate the consumption of H2O2 by VMn-Gd, titanium sulphate (H2TiO4) was used as an indicator since H2TiO4 can be degraded by H2O2 accompanied by the production of yellow products (Ti(SO4)2) (Fig. 2Gi, Gii, S4). Interestingly, data showed that the exhaustion of H2O2 had the concentration of VMn-Gd dependent manner, especially when pH value was set at weak acid condition with 2.5 times higher H2O2 decomposition efficiency than that of pH 8.5 group (Fig. 2H). The concentration of H2O2 reached equilibrium at these three pH conditions when the concentration of VMn-Gd was set as 0.5 mg mL−1. In addition, VMn-Gd and H2O2 can quickly reach saturation within ~ 4 h at pH 6.5, while, the reaction rate significantly decreased and the saturated time was ~ 6 h at pH 7.4 group. However, at pH 8.5, there is indiscernible H2O2 decomposition (Fig. 2I). Moreover, MnO2 can also react with intracellular GSH to produce Mn2+. Hence, to evaluate exhaustive ability of VMn-Gd@GOx toward GSH under different pH values, here, 5,5’-Dithiobis-(2-nitrobenzoic acid) (DTNB) was used as an indicator since DTNB can be degraded by GSH accompanied by the production of yellow products (TNB) (Fig. 2Ji, Jii, S5). Obviously, we found that VMn-Gd@GOx and VMn-Gd exhibit the same tendency to consume GSH that overwhelmingly higher than PBS treated group (Fig. S6). Further, results also showed that, in contrast with pH 7.4 and pH 8.5 groups, GSH can be significantly decomposed by VMn-Gd@GOx at pH 6.5, especially within 10 min of incubation (Fig. 2K-M, S7). In brief, all above findings demonstrated that our Mn-Gd based nanoplatform can be applied for MRI contrast agent and it is able to induce ROS storm under X-ray, high glucose as well as high GSH conditions. This programmed nanoagents held great potential for the following tumor tissues diagnosis and suppression via synergistic RT and oxidative therapy.
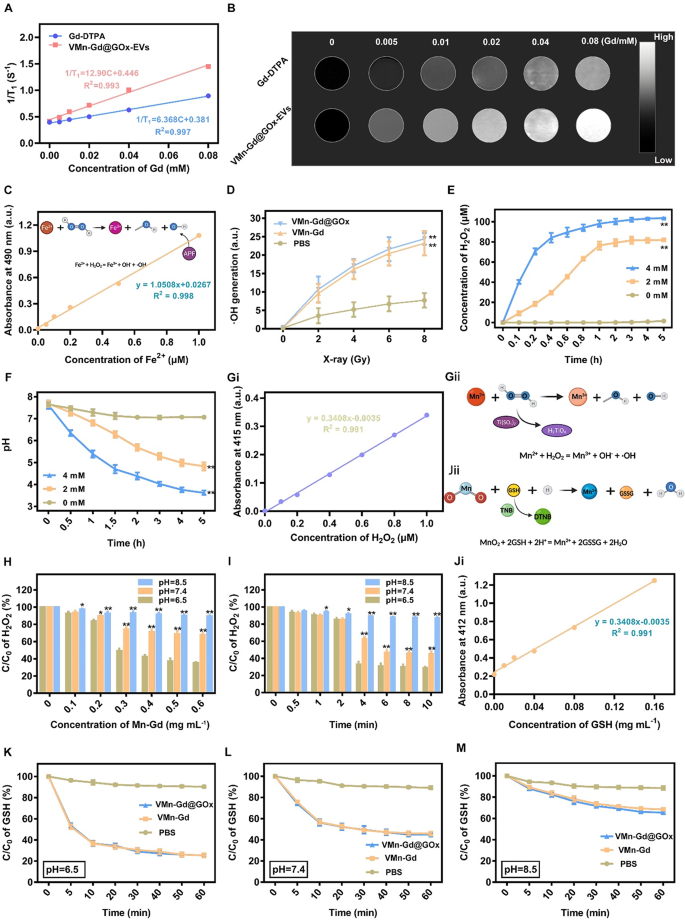
The r1 relativities (A) and in vitro MRI images (B) of Gd-DTPA and VMn-Gd@GOx under different concentrations. (C) Standard curve of AFP vs. various concentrations of Fe2+ for evaluating •OH generation under X-ray irradiation. (D) Assessments of •OH generation in VMn-Gd@GOx, VMn-Gd and PBS under various power density of X-ray. (E) The generated H2O2 concentrations and (F) fluctuation of pH values after incubated VMn-Gd@GOx with different dose of glucose for various durations. (Gi) Standard curve of Ti(SO4)2 vs. various concentrations of H2O2 for evaluating •OH generation under Mn ions catalysis. Schematic illustration of (Gii) H2TiO4 for •OH detection and (Jii) DNTB for GSH consumption. Residual H2O2 percentages after incubated with VMn-Gd@GOx (H) at different dose or (I) for various periods under pH 6.5, pH 7.4 and pH 6.5 conditions. (Ji) Standard curve of TNB vs. various concentrations of GSH. The residual GSH content after treating VMn-Gd@GOx, VMn-Gd and PBS with (K) pH = 6.5, (L) pH = 7.4 and (M) pH = 8.5 buffers for different minutes. The data are presented as the mean ± SD (n = 3), with P-values determined by one-way ANOVA followed by Bonferroni correction. *P P
In vitro tumor cell killing efficacies of Mn-Gd@GOx-EVs
We were motivated to investigate the therapeutic mechanism and efficacy of VMn-Gd@GOx-EVs at the cellular level (Fig. 3A). Before assessing the cytotoxic effects induced by X-rays, we evaluated the intracellular uptake of these therapeutic nanomaterials using the 4T1 breast carcinoma cell line. In this case, GOx was replaced by the fluorophore Ce6, resulting in the formation of VMn-Gd@Ce6-EVs. This allowed the visualization of the internalization process via CLSM and flow cytometry. As shown in Fig. 3B, C and S8, the intracellular red fluorescence of VMn-Gd@Ce6-EVs was distinctively higher than that of free Ce6 and VMn-Gd@Ce6 (Ce6-labeled VMn-Gd) at all tested time points (6, 12, and 24 h). Furthermore, the endocytosis efficiency of VMn-Gd@Ce6 was significantly higher compared to free Ce6. This improvement can be attributed to the virus-inspired rough surface of VMn-Gd@Ce6, which strongly interacts with cell membranes, facilitating the entry of Ce6 into the cells. Additionally, the EVs coating enhanced cellular endocytosis, highlighting the potential of EVs as effective vehicles for intercellular delivery. The superior compatibility of the EVs with the cell membrane might be the key factor driving this enhanced internalization. These findings further confirmed that VMn-Gd@GOx-EVs is an optimal formulation for efficient nanotherapeutic delivery and shows great promise as a biomimetic drug delivery system. After establishing the endocytic properties of the nanomaterials, we evaluated the cytotoxicity of VMn-Gd@GOx-EVs under X-ray irradiation using a cell counting kit-8 (CCK-8) assay. As seen in Fig. 3D, PBS-treated tumor cells exhibited undiscoverable toxicity upon X-ray exposure, demonstrating the biosafety of X-rays at the tested power density (6 Gy). In contrast, cells exposed to VMn-Gd-based formulations exhibited increased cytotoxicity in a dose-dependent manner. Notably, the VMn-Gd@GOx-EVs group showed the highest cell killing ability compared to the VMn-Gd and VMn-Gd@GOx groups when all groups received X-ray illumination. This enhanced cytotoxicity could be attributed to the strong endocytic activity of the EVs. Additionally, the cytotoxic effect was further amplified by increasing the X-ray power density (Fig. 3E). At 6 Gy, 73.88% of cells in the VMn-Gd@GOx-EVs group (10.88 µg•mL−1 Gd equivalent) were killed. This X-ray power density was used in all subsequent in vitro and in vivo studies for tumor cell elimination. Moreover, apoptosis analysis was performed using the Annexin V-FITC/PI co-staining assay (Fig. 3G). The results showed no detectable apoptosis in PBS, VMn-Gd, or Mn-Gd@GOx-EVs-treated tumor cells, confirming the biocompatibility of these formulations in vitro. VMn-Gd + X-ray treatment also exhibited a moderate cell killing effect (38.29%), indicating notable radiotherapy efficiency. Importantly, VMn-Gd@GOx-EVs treated with X-ray irradiation showed the highest apoptosis rate (61.20%), which aligned with the previous CCK-8 results (Fig. 3F). In summary, VMn-Gd oxidative nanoagents could exhausted GSH for manganese ions (Mn2+) and O2 generation, thereby enhancing RT efficiency for •OH production. GOx can induce pH decline and H2O2 increase that subsequent •OH generated under Mn2+ catalysis. Therefore, ROS storm was produced in cytoplasm for effective tumor cell killing via synergistic RT and oxidative therapy.
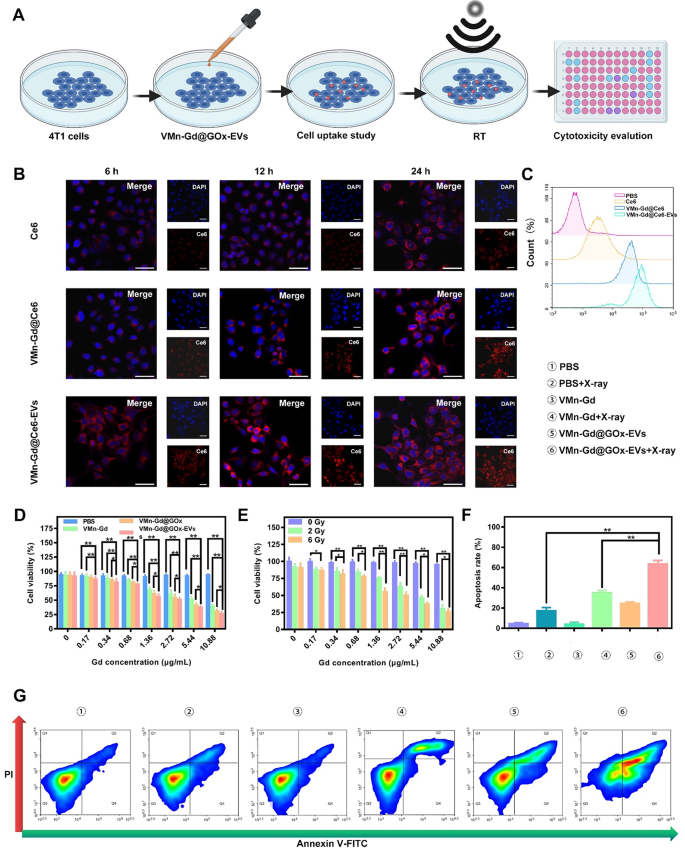
(A) Schematic representation of the treatment protocol for VMn-Gd@GOx-EVs in 4T1 cells. (B) CLSM images showing 4T1 cells after incubation with free Ce6, VMn-Gd@Ce6, or VMn-Gd@Ce6-EVs for 6, 12, and 24 h, all at equivalent Gd concentrations. The amount of Ce6 was matched across all groups, including free Ce6, VMn-Gd@Ce6, and VMn-Gd@Ce6-EVs. Scale bar represents 30 μm. (C) Flow cytometry analysis of cellular uptake in 4T1 cells after 24-hour exposure to free Ce6, VMn-Gd@Ce6, or VMn-Gd@Ce6-EVs under the same Gd concentration. (D) Cell viability assessment of 4T1 cells post-treatment with PBS, VMn-Gd, VMn-Gd@GOx, or VMn-Gd@GOx-EVs under X-ray irradiation (6 Gy) across various Gd concentrations. (E) Evaluation of the cytotoxicity of VMn-Gd@GOx-EVs at different Gd levels with varying doses of X-ray (0, 3, and 6 Gy). (F-G) Quantification of apoptosis in 4T1 cells following exposure to different treatment formulations using Annexin V-FITC/PI dual staining. Results are shown as mean ± SD (n = 3). Statistical significance was analyzed using one-way ANOVA followed by Bonferroni correction: *P P
Undoubtedly, GOx specifically catalyzes the oxidation of β-D-glucose under high O2 condition. Therefore, to assess the catalytic activity of GOx, a hypoxia-inducible factor 1-alpha (HIF-1α) probe was employed to evaluate the extent of hypoxia at the cellular level. As shown in Fig. 4A, in stark contrast to the minimal levels of HIF-1α detected in normoxia and PBS-treated 4T1 cells, a strikingly intense red fluorescence signal was observed in cells subjected to incubation with VMn-Gd@GOx-EVs for 12 h with X-ray irradiation, profoundly demonstrating the strikingly high glucose consumption. This compelling observation indicated a significant up-regulation of intracellular HIF-1α expression within the hypoxic milieu. Nevertheless, a significantly reduced expression of HIF-1α was then discerned in cells subjected to incubation with VMn-Gd@GOx-EVs plus X-ray shining for more than 24 h (Fig. 4B). These findings can be attributed to massive Mn ions release and high GSH levels in tumor microenvironment, mediating O2 generation. In addition, we meticulously assessed the expression levels of HIF-1α, employing Western blot analysis for precise evaluation. As illustrated in Fig. 4G, the lowest concentration of HIF-1α within the cytoplasm was prominently observed in the VMn-Gd@GOx-EVs group subjected to X-ray irradiation, aligning seamlessly with trends identified in prior studies. This observation underscored a remarkable mitigation of hypoxia, attributable to the O2 generation facilitated by VMn-Gd catalysis. Importantly, hypoxia relief with HIF-1α down-regulation always has positive correlation with radio-sensitization that induced the increase in ROS production levels, accompanying by enhanced tumor cell toxicity via DNA damage-induced cell death pathways. Following closely, the phosphorylated protein γ-H2AX is specifically localized at sites of DNA double-strand breaks and is esteemed as a pivotal biomarker for DNA damage induced by ionizing radiation. Consequently, to further evaluate the radio-sensitization potential of VMn-Gd@GOx-EVs, the levels of γ-H2AX within cellular nuclei were meticulously assessed utilizing a DNA damage assay kit, followed by visualization through CLSM. As illustrated in Fig. 4C, the VMn-Gd@GOx-EVs combined with X-ray treatment exhibited a markedly more intense green fluorescence compared to other formulations. Particularly, the quantitative assessment of DNA damage levels within this group was significantly elevated when juxtaposed with both the PBS + X-ray and the standalone VMn-Gd@GOx-EVs groups. The density of γ-H2AX, as shown in Fig. 4D, underscores the significant DNA damage induced by the VMn-Gd@GOx-EVs + X-ray group. Furthermore, radio-cytotoxicity typically mediates DNA fragmentation and obstructs DNA replication, thereby fundamentally impeding clonal cell proliferation. Here, the formation of colonies is illustrated in Fig. 4E, F. The VMn-Gd@GOx-EVs combined with X-ray treatment demonstrated a remarkable ability to diminish the clonal formation rate to approximately 7.73%. This represents a significant reduction, being 2.28-fold and 5.09-fold lower than that observed in the PBS + X-ray group (approximately 21.93%) and the single Mn-Gd@GOx-EVs group (around 38.33%), respectively. We were prompted to assess the expression levels of γ-H2AX, alongside the proliferation-associated protein c-myc, which were meticulously evaluated through Western blot analysis. As anticipated, the highest concentration of γ-H2AX and the lowest expression of c-myc within the cytoplasm were distinctly observed in the VMn-Gd@GOx-EVs subjected to X-ray irradiation (Fig. 4G, H-J, S9). These findings align seamlessly with trends established in prior research. Briefly, all data further substantiated that VMn-Gd@GOx-EVs exhibited a remarkable radio-sensitization effect, significantly impeding the proliferation of cancerous cells. These positions themed as a promising candidate for nanoagents of radio-sensitization therapy.
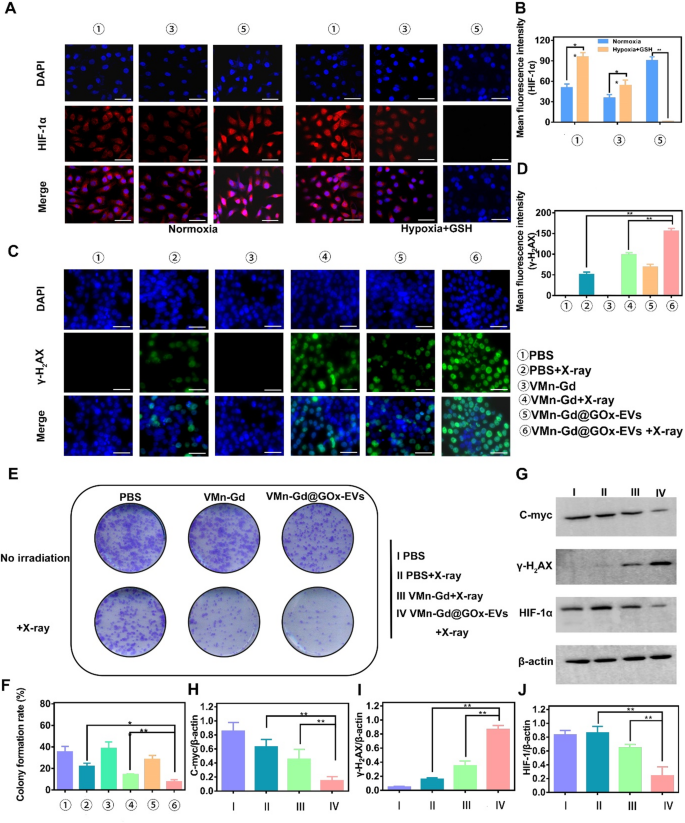
(A) CLSM images and quantitative fluorescence intensities (B) of HIF-α in 4T1 cells treated with different formulations. Cells were cultured under normoxic conditions (left) and hypoxia with high GSH conditions (right). (C) CLSM images and (D) average fluorescence intensity (γ-H2AX) of 4T1 cells treated with various formulations to assess DNA fragmentation and nuclear condensation using γ-H2AX staining. (E) Representative images and (F) colony formation rate from a colony formation assay. (G) Impact of VMn-Gd@GOx-EVs combined with X-ray irradiation (6 Gy) on the expression levels of γ-H2AX, HIF-1α, and C-myc proteins in 4T1 cells. Corresponding γ-H2AX/β-actin, HIF-1α/β-actin, and C-myc/β-actin ratios in 4T1 cells following various treatments. Scale bar represents 100 μm. The data are presented as the mean ± SD (n = 3), with P-values determined by one-way ANOVA followed by Bonferroni correction. *P P
To further assess tumor cell killing, a live/dead cell staining assay was conducted using VMn-Gd@GOx-EVs combined with X-ray irradiation. Consistent with the apoptosis data, most of the tumor cells were stained with red fluorescence, indicating cell death. PBS, VMn-Gd, and VMn-Gd@GOx-EVs groups showed minimal toxicity, with cells emitting green fluorescence, confirming minimal impact on normal cells (Fig. 5A). In the VMn-Gd + X-ray group, however, significant numbers of dead cells were observed, consistent with the apoptosis results, suggesting that the single RT approach had some limitations in eradicating malignant cells. In contrast, VMn-Gd@GOx-EVs combined with X-ray irradiation exhibited strong tumor-specific cytotoxic effects, driven by a combination of ROS generation from tumor cell targeting, RT, a Fenton-like reaction and GSH depletion. Building on these findings, the generation of intracellular ROS was further examined using DCFH-DA staining (Fig. 5B). CLSM images revealed that VMn-Gd@GOx-EVs with X-ray exposure produced a significantly higher green fluorescence, approximately 6 times greater than the ROS levels in the PBS group with X-ray irradiation (Fig. 5C). Prominently, ROS levels were higher in the VMn-Gd@GOx-EVs + X-ray group compared to the VMn-Gd@GOx + X-ray group. The precise tumor tissues targeting of EVs, the reduction of pH and the generation of H2O2 by GOx that fundamentally promoted the Fenton-like reaction of Mn ions. Further, both GSH consumption and hypoxia alleviating had enhanced the radiotherapy efficiency upon X-ray irradiation. Consequently, compared to other treatment strategies, all above advantages contributed to the efficient cell killing via ROS storm in VMn-Gd@GOx-EVs + X-ray group. Mitochondria present an ideal target for enhancing ROS-based attacks in cancer treatment. Nanocarriers with a positive charge are considered optimal for targeting mitochondria. By leveraging the unique characteristics of mitochondria, the targeted delivery of Mn2+ to this organelle can effectively maximize the generation of ROS for oxidative therapy. To visualize this, we used the JC-1 probe, which aggregates and emits red fluorescence when the mitochondrial membrane potential is intact. Upon depolarization, it transitions to a monomeric form, emitting green fluorescence. As shown in Fig. 5D, E, the red fluorescence gradually decreased while the green fluorescence increased in cells treated with VMn-Gd@GOx-EVs followed by X-ray irradiation. Importantly, the red/green fluorescence intensity ratio was notably reduced in the VMn-Gd@GOx-EVs + X-ray group compared to the other groups. These CLSM results confirm that the Mn-Gd bimetallic nanoplatform effectively induces ROS generation under X-ray irradiation and Fenton-like catalysis, leading to significant cytotoxic effects on tumor cells.
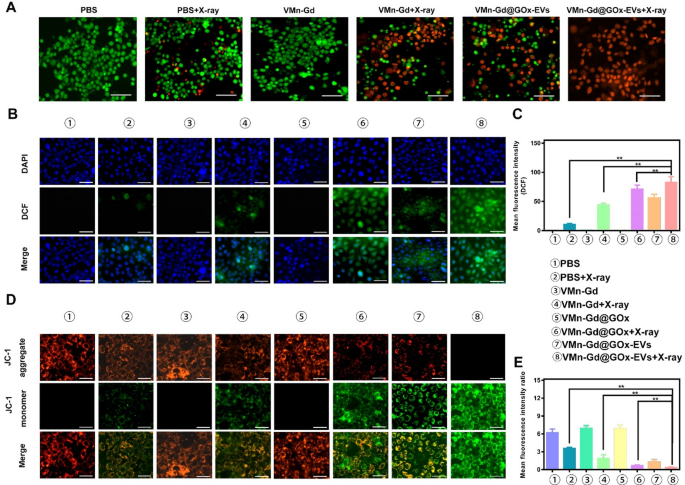
(A) Live-dead cell staining of 4T1 cells treated with various formulations and X-ray irradiation (6 Gy) using calcein-AM/PI assay (scale bar represents 60 μm). (B) CLSM images and (C) corresponding quantitative fluorescence intensities of 4T1 cells treated with different formulations, followed by X-ray irradiation (6 Gy), to assess ROS generation via DCFH-DA staining (scale bar represents 30 μm). (D) CLSM images and (E) quantitative fluorescence intensities of 4T1 cells treated with various formulations under X-ray exposure (6 Gy) to evaluate mitochondrial membrane potential using JC-1 staining (scale bar represents 30 μm). The data are presented as the mean ± SD (n = 3), with P-values determined by one-way ANOVA followed by Bonferroni correction. *P P
In vivo breast cancer targeting
The encouraging radiotherapy and tumor targeting capabilities observed in vitro prompted an in vivo study to assess the therapeutic potential of Mn-Gd@GOx-EVs. In this study, 4T1 cells, a murine breast carcinoma model known for rapid malignant growth and extensive multi-organ metastasis in BALB/c mice, were used. Subcutaneous 4T1 tumor-bearing mice were chosen for this experiment. To monitor the in vivo biodistribution and tumor targeting ability of VMn-Gd@ICG-EVs, we replaced GOx with Indocyanine green (ICG), a near-infrared (NIR) dye, in the virus-like nanoparticles (VMn-Gd@ICG-EVs). Fluorescence imaging was performed using a 50 ms exposure time, 808 nm laser excitation, and a 1000 nm long-pass filter. As shown in Fig. 6A, the initial tumor location was clearly distinguishable from surrounding muscle tissue at 6 h post-administration of VMn-Gd@ICG-EVs. The fluorescence intensity significantly increased, reaching its peak at 12 h after tail-vein injection. At 24 h, the NIR signal in the tumor began to fade, with complete attenuation observed at 36 h (Fig. 6A). In contrast, the ICG-only group showed much weaker fluorescence at the tumor site throughout the imaging period with three-fold lower NIR intensity than VMn-Gd@ICG-EVs treated group at 24 h (Fig. S10). In this study, Mn/Gd ions typically are triggered to release under weak acid tumor microenvironment. These released ions can effectively interact with surrounding water molecules in tumor tissues, altering the local magnetic resonance properties, thereby enhancing the MRI signal. In light of this, the in vivo tumor targeting ability was assessed over a 24-hour period using MRI after the administration of VMn-Gd@GOx-EVs via the tail vein. Strong T1-weighted MR signals were observed at the tumor site, starting at 3 h post-injection, and reached peak intensity at 12 h (Fig. 6B, C), consistent with the NIR fluorescence imaging results (Fig. 6A). Markedly, even at 24 h, a significant MR signal remained around the tumor area, confirming that VMn-Gd@GOx-EVs effectively accumulated in the tumor microenvironment (Fig. 6B, C). This finding suggests that the Mn-Gd-based nanoplatform can serve as an MRI contrast agent for potential clinical applications. Based on these observations, X-ray irradiation for RT should be conducted 12 h after nanoplatform injection to achieve optimal tumor suppression (Fig. 6D).
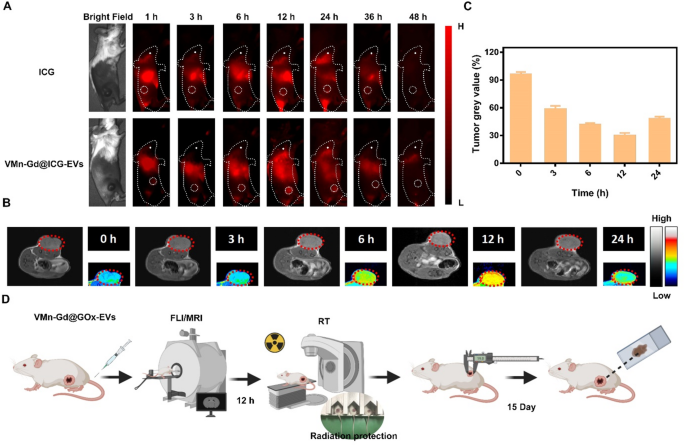
(A) In vivo NIR fluorescence imaging of 4T1 tumor-bearing mice at various time points following the injection of either VMn-Gd@ICG-EVs or ICG. (B) In vivo MRI scans and tumor grayscale values of 4T1 tumor-bearing mice at various time points after injection of VMn-Gd@GOx-EVs. (C) Tumor grey value at different time intervals after treatment with VMn-Gd@ICG-EVs. (D) A flowchart for in vivo tumor imaging followed by tumor treatments. The data are presented as the mean ± SD (n = 4), with P-values determined by one-way ANOVA followed by Bonferroni correction. *P P
In vivo radiosensitization therapy of antitumor effect of VMn-Gd@GOx-EVs with X-ray irradiation
Finally, the therapeutic efficacy of radiosensitization therapy utilizing VMn-Gd@GOx-EVs in conjunction with X-ray irradiation was meticulously assessed through the establishment of a subcutaneous transplantation model in BALB/c mice. A series of three tail-vein injections comprising various formulations were administered on days 1, 3, and 5. Following this regimen, X-ray exposure (6 Gy) was executed on the tumors within three of these groups at 12 h post-injection (Fig. S11). The tumor growth volumes were monitored every three days, clearly, the parallelism of tumor inhibition trends was tracked. As illustrated in Fig. 7A-C and S12, the tumors within the PBS and VMn-Gd cohorts exhibited a rapid proliferation. In stark contrast, all VMn-Gd-based groups subjected to X-ray irradiation demonstrated a notable degree of tumor growth inhibition, indicating that the ROS production induced by X-ray irradiation could significantly impede the proliferation of tumor masses. Strikingly, in comparison to the PBS + X-ray and VMn-Gd + X-ray cohorts, the VMn-Gd@GOx-EVs + X-ray group demonstrated a markedly enhanced inhibitory effect on tumor proliferation, exhibiting the smallest tumor sizes. This remarkable outcome can be attributed to the radiosensitization properties of VMn-Gd combined with the EVs’ camouflage and the O2 generation that facilitated prominently enhanced the RT efficiency. Furthermore, it is particularly noteworthy that GOx and GSH depletion significantly amplified ROS catalyzed efficacy while concurrently impeding some extent of tumor growth in VMn-Gd@GOx-EVs treated mice. The staining of Ki67, CD31, and HIF-1α is esteemed as pivotal assays for evaluating the efficacy of radiation therapy. In this context, we conducted immunohistochemical staining on sections of tumor tissue. The results were strikingly evident in the Ki67-stained slides (Fig. 7D). Specifically, when juxtaposed with alternative formulations, a remarkable reduction ranging from two to fourfold in Ki67 levels was observed within malignant breast tumors following the treatment with VMn-Gd@GOx-EVs combined with X-ray exposure (Fig. 7E). Furthermore, radiation possesses the remarkable ability to orchestrate the obliteration of blood vessels within tumors, thereby impeding tumor proliferation. The presence of intratumoral blood vessels is frequently inferred from the expression of CD31 on the surface of endothelial cells. As illustrated in Fig. 7D, F, the proportion of CD31-positive cells within the tumors of the VMn-Gd@GOx-EVs + X-ray cohort was markedly diminished compared to those observed in other experimental groups. These findings compellingly demonstrate that the combination of VMn-Gd@GOx-EVs and X-ray irradiation can effectively obliterate intratumoral blood vessels, thereby obstructing the vascular supply within the tumor and impeding its continued proliferation. In addition, the VMn-Gd@GOx-EVs + X-ray demonstrated a remarkable capacity to alleviate hypoxia, thereby significantly augmenting the efficacy of radiotherapy. We further investigated the expression levels of HIF-1α. As illustrated in Fig. 7D, G, the proportion of intra-tumoral HIF-1α-positive cells within the VMn-Gd@GOx-EVs + X-ray group was conspicuously lower than that observed in the other experimental groups. These findings imply that the radiosensitizing effect facilitated by VMn-Gd@GOx-EVs could substantially enhance the tumor ablation outcomes of unparalleled ROS storm via RT and Fenton-like catalysis. Meanwhile, the increased ROS levels in the cytoplasm are critical for inducing immunogenic cell death, where apoptotic tumor cells release tumor-associated antigens that activate dendritic cells (DC) maturation, ultimately helping to prevent tumor recurrence and metastasis [46,47,48,49]. To evaluate this process, the expression of CD80 and CD86, markers of DC maturation in the tumor, was analyzed using flow cytometry. Remarkably, the results demonstrated that the highest levels of CD80+/CD86+ DC were observed following treatment with VMn-Gd@GOx-EVs + X-ray, surpassing all other treatment groups (Fig. S13), indicating that this approach effectively promotes DC maturation that can be further applied for immunotherapy.
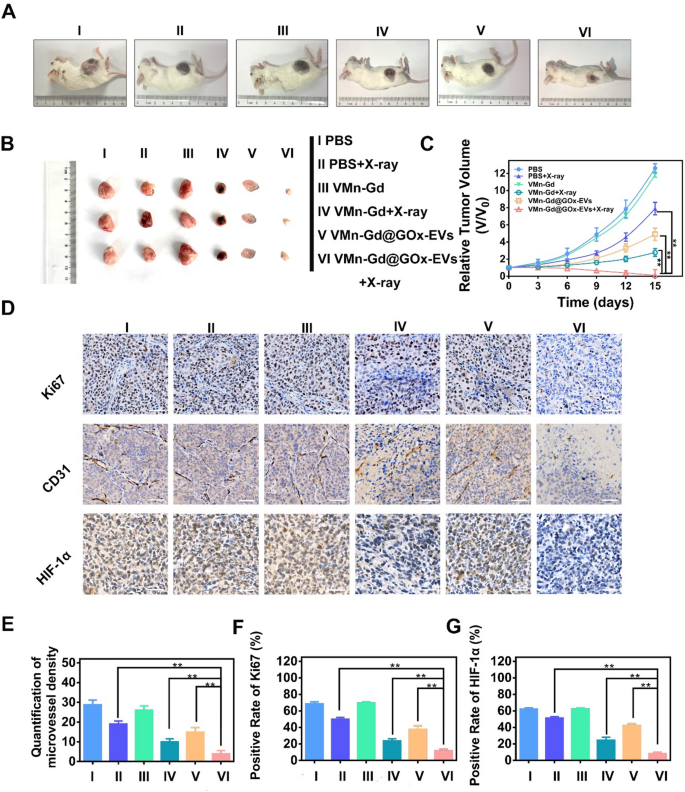
(A) Representative photographs of 4T1 tumor-bearing mice on the 15th day after different treatments. (B) Representative photographs of solid tumors after different treatments. (C) Tumor volume variations after different treatments for 15 days. (D) Immunohistochemical staining of Ki67, CD31 and HIF-1α levels in different treated tumor tissues at day 7. (E) Quantification of microvessel density, (F) Positive rate of Ki67, and (G) positive rate of HIF-1α after different administrations at day 7. The data are presented as the mean ± SD (n = 4), with P-values determined by one-way ANOVA followed by Bonferroni correction. *P P
Biosafety evaluation
Biosafety represents a paramount concern in the preclinical application of nanotherapeutics. Consequently, comprehensive assessments were undertaken, encompassing body weight measurements, histological examination of major organs via H&E staining, as well as routine serum analyses and biochemical evaluations. As shown in Fig. 8A, significantly tumor cell damage was found in VMn-Gd@GOx-EVs + X-ray treated tumor, exhilaratingly, negligible tissue damage and fragmented cell membranes were observed in the principal organs, including the heart, liver, spleen, lungs, and kidneys (Fig. S14). Furthermore, none of the mice exhibited any significant loss in body weight throughout the entire duration of the treatment period (Fig. 8B). To further ascertain whether VMn-Gd@GOx-EVs + X-ray induced acute toxicity, blood samples were meticulously collected from mice on the 15th day following treatment for comprehensive biosecurity analysis. As illustrated in Fig. 8C-E and S15, the principal serum enzyme levels (including AST, ALT, and CRE) as well as blood cell counts encompassing WBC, NE, LY, RBC, HGB, and PLT were consistently maintained within standard physiological ranges. Collectively, these findings underscored the negligible systemic toxicity associated with the VMn-Gd@GOx-EVs plus X-ray treatment group. Subsequently, we carefully monitored the survival rates of mice subjected to various therapeutic interventions. As shown in Fig. 8F, nearly all BALB/c mice bearing mammary tumors in the PBS group succumbed by day 30 post-treatment. In stark contrast, the survival rate of mice administered with VMn-Gd@GOx-EVs combined with X-ray treatment was significantly extended beyond 60 days. This stands in marked comparison to the PBS + X-ray group, which exhibited a survival duration of merely 40 days. This remarkable finding can be attributed to the commendable ROS storm for tumor eradication. Furthermore, to elucidate the in vivo biodistribution of VMn-Gd@GOx-EVs, we meticulously harvested the principal organs and tumors from mice following intravenous administration of nanoparticles at various time intervals. The concentrations of Gd3+ were subsequently quantified using inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 8G, S16). During the initial 6 h following intravenous injection, VMn-Gd@GOx-EVs predominantly localized within the liver and spleen (Fig. 8H, S17-S21). In contrast, a substantial quantity of nanoagents exhibited significant accumulation at the tumor site, peaking approximately at the 12-hour mark (Fig. S22). Subsequently, there was a notable decline in VMn-Gd@GOx-EVs across the examined organs as time progressed. In parallel, to elucidate the excretion dynamics of VMn-Gd@GOx-EVs in murine models, we quantified the levels of Gd3+ present in feces and urine collected post-injection of nanoparticles at various intervals. In the initial stages, VMn-Gd@GOx-EVs that accumulated in the liver were predominantly excreted via bile into feces. By the 17th day, the degraded nanoparticles could be eliminated through the kidneys, resulting in urine formation (Fig. 8I). On the fourteenth day, a peak was observed in nanoparticle excretion through urine. Subsequently, this discharge gradually diminished and by the 28th day, approximately 95% of VMn-Gd@GOx-EVs were expelled at an elevated rate through both feces and urine (Fig. S23, S24). These phenomena suggested that VMn-Gd@GOx-EVs also exhibited inherent biodegradability under physiological condition for long time durations. EVs are able to escape detection and clearance by phagocytic immune cells, while simultaneously being efficiently taken up by the intended target cells or tissues. Cytokine changes (including IL-6, TNF-α and IL-1β) in blood were tested after tail vein administration of VMn-Gd@GOx-EVs for different periods. Exhilaratingly, in sharp contrast with normal group, indiscernible cytokine fluctuations were discovered even after 48 h postinjection (Fig. S25), profoundly demonstration the unparalleled immune evasion ability after EVs camouflage. All results demonstrated that VMn-Gd@GOx-EVs, when subjected to X-ray irradiation, could elicit a highly favorable ROS generation for tumor elimination, all while exhibiting negligible long-term side effects. This underscores their exceptional biological safety and presents an optimal therapeutic outlook for oncological applications. This study also has the following limitations, such as insufficient exploration of immune-mediated cell death, lack of investigation into the blood half-life, and the absence of short-term acute toxicity of VMn-Gd@GOx-EVs at high concentration. Moreover, it has been reported that released Gd3+ can bind with ATP in cytoplasm [50], and further investigation is needed to determine whether our nanoplatform induces an energy deprivation effect within cells.
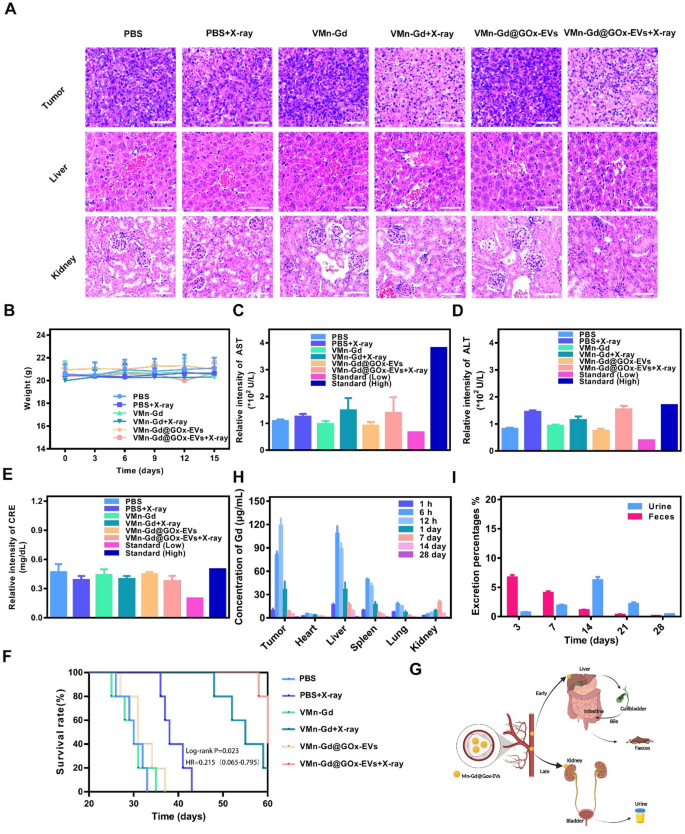
(A) H&E stained images of tumor and vital organs after various treatments. (B) Body weight fluctuated curves during the tumor suppression period. (C) ALT, (D) AST and (E) CRE levels in different groups. (F) Survival rates of six different groups for 60 days. (G) Diagrammatic illustration of excretion percentage from urine and faeces after tail-vein injection of VMn-Gd@GOx-EVs for different periods. (H) Bio-distribution of VMn-Gd@GOx-EVs after tail-vein injection for different periods. (I) Excretion percentage of VMn-Gd@GOx-EVs after tail-vein injection for different periods. The data are presented as the mean ± SD (n = 4), with P-values determined by one-way ANOVA followed by Bonferroni correction. *P P


