Materials
All the chemical agents were analytical reagents (AR). Cerium acetate (Ce(Ac)3), sodium hydroxide (NaOH) and 2,2-azino-bis(3-ethyl-benzothiazole-6-sulfonic acid) diammonium salt (ABTS) were obtained from Aladdin Reagent Co., Ltd (Shanghai, China). Sulfasalazine (SASP) was purchased from Shanghai McLean Biochemical Technology Co., Ltd (Shanghai, China). Soluble starch was purchased from China National Pharmaceutical Group Co., Ltd (Beijing, China). FeSO4·7H2O was acquired from Sigma Aldrich (Shanghai, China). Hydrochloric acid (HCl) and methylene blue (MB) were purchased from the Tianjin Kermel Chemical Reagent Company (Tianjin, China). Hydrogen peroxide (H2O2, 30%) was purchased from Sichuan Xilong Science Co., Ltd (Sichuan, China). Dulbecco’s modified eagle medium (DMEM), phosphate buffered solution (PBS) and fetal bovine serum (FBS) were obtained from Wuhan Prosa Life Science & Technology Co., Ltd (Wuhan, China). Cell Counting Kit-8 (CCK-8) cytotoxicity assay kit and RAW264.7 cells were gifted by Henan Shengxiang Pharmaceutical Technology Co., Ltd (Henan, China). Anti-tumor necrosis factor-α (TNF-α), anti-chemokine interleukin-1β (IL-1β), anti-chemokine interleukin-10 (IL-10) were purchased from Solarbio Technology Co., Ltd (Beijing, China). HT29 cell and medium purchased from Keycell Biotechnology Co., Ltd (Wuhan, China). Escherichia coli of strain ATCC25922 was used.
Synthesis of Ce-SASP-RS ICPs
Firstly, resistant starch was obtained by dispersing 80 mg soluble starch in 100 mL of boiling water with keeping for 30 min, followed by cooling to room temperature. Then SASP was added into 10 mL of starch solution (0.08%) containing 0.25 mM NaOH. After SASP dissolved in the above solution, 10 mL of starch solution (0.08%) was further added to dilute the solution. Subsequently, 20 mL of starch solution (0.08%) containing Ce(Ac)3 was added dropwise to the above solution under stirring conditions and kept stirring for 30 min. Finally, the products were collected by centrifugation at 7500 rpm, washing with distilled water and drying at 80 ℃.
Characterization
The powder X-ray diffraction (XRD) patterns were collected on an X-ray diffractometer (D8-Advance) equipped with Cu-Kα radiation. The morphology of the as-synthesized samples was observed using a transmission electron microscope (TEM, FEI Tecnai G2 20) and scanning electron microscope (SEM, FEI Quanta 250 FEG). The UV–vis absorption spectra were recorded on a Hitachi U4100 spectrophotometer (Hitachi, Japan). The FTIR spectra were acquired by FTIR spectrometer (Thermo Scientific Nicolet iS5). The concentration of cerium ions was measured using inductively coupled plasma-atomic emission spectroscopy (iCAP 7600 ICP-OES, ThermoFisher). The zeta potential was characterized with Malvern zetasizer Nanoseries (Nano ZS90). X-ray photoelectron spectroscopy of C 1 s, O 1 s and Ce 3d of the samples were obtained from ESCAlab250, Thermal Fischer.
Measurement of superoxide dismutase (SOD)-like activity
The SOD-like activities of Ce-SASP-RS ICPs and SASP were evaluated by using 2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium Sodium Salt (WST-8) assay kit. Firstly, 40 μL of each material with different final concentration (12.5, 25, 50, 100, 200 μg/mL) was mixed with 320 μL of WST-8/xanthine oxidase working solution. Then, 40 μL of working solution for initiating the reaction was added into the above system. After 30 min of incubation at 37 ℃, the absorbance of solution at 450 nm was measured by UV–Vis spectrophotometer.
Measurement of peroxidase (POD)-like activity
The POD-like activities of SASP and Ce-SASP-RS ICPs were evaluated by using 2,2’-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) as substrate in the presence of H2O2. In detail, samples with different final concentrations (50, 100, 200, 300, 400, 500 μg/mL) were mixed with solution containing 100 μL of H2O2 (30%) and 300 μL of ABTS (10 mg/mL) (final volume was 4 mL) at room temperature. After the chromogenic reactions, the absorbance of the solution at 740 nm was measured by UV–vis spectrophotometer.
Measurement of hydroxyl radical scavenging activity
The •OH scavenging ability of Ce-SASP-RS ICPs and SASP was measured by using methylene blue (MB) as indicator, which can be discolored by •OH and its absorption peak at 664 nm can be decreased. And Fenton reaction between H2O2 and FeSO4 was employed to generate •OH. Specifically, Ce-SASP-RS ICPs solution with different concentrations was added to a solution containing 40 μL of MB (1 mg/mL), 80 μL of FeSO4 (10 mM) and 180 μL of H2O2 (10 mM), and the final volume of the system was fixed at 4 mL with distilled water. The mixture solution was kept standing for 3 min and the remaining •OH was quantified by UV–vis absorption spectroscopy. The scavenging rate of •OH was calculated using the following formula:
scavenging rate (%) = [(A2-A1)/(A0-A1)] × 100%,
where A0 was the absorbance of pure MB, A1 and A2 were the absorbance of MB incubated in the Fe2+/H2O2 fenton system without and with Ce-SASP-RS ICPs, respectively.
Measurement of cerium ions release
The stability of Ce-SASP-RS ICPs in solution with different pH values was evaluated by using ICP-OES to detect the released amount of cerium ions from Ce-SASP-RS ICPs. Briefly, dialysis bag containing 3 mg of Ce-SASP-RS ICPs was placed in 100 mL of buffer solutions with different pH values (pH ~ 1.2, 4.0, 6.0, 7.4. 8.0). At predetermined time points, 8 mL of the buffer solution was taken out for measuring the released cerium ions with ICP-OES.
Cell experiments
Cell culture
Mouse mononuclear macrophage leukemia cells (RAW264.7 cells) were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin at 37 ℃ in humidified atmosphere with 5% CO2. Human colorectal adenocarcinoma cell line (HT29 cells) was cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin at 37 ℃ in humidified atmosphere with 5% CO2.
Cytotoxicity experiment
Cytotoxicity experiments were performed with the CCK-8 kit. RAW264.7 cells and HT29 cells were seeded in 96-well plates at a cell density of 1 × 104 per well and incubated for 24 h. The medium was then replaced with fresh medium containing Ce-SASP-RS ICPs or SASP at different concentrations and incubated with cells for another 24 h. After that, 10 μL of CCK-8 was added into each well and kept further incubation for 1 h at 37 ℃. Finally, the absorbance of supernatants at 450 nm was detected with a microplate reader.
In addition, the protective effect of Ce-SASP-RS ICPs in ROS environment was investigated with the similar method. In detail, RAW264.7 cells were seeded in a 96-well plate at a cell density of 1 × 104 per well and incubated for 24 h. Then, the medium was removed, and new fresh medium containing Ce-SASP-RS ICPs or SASP (200 μg/mL), with or without H2O2 (1.5 ~ 3 mM), were added into each well for another 24 h of incubation. Subsequently, 10 μL of CCK-8 was added into each well and kept further incubation for 1 h at 37 ℃. Finally, the absorbance of supernatants at 450 nm was detected with a microplate reader.
Measurement of intracellular ROS
The fluorescent probe 2,7-Dichrorodihydrofluorescein diacetate (DCFH-DA) was employed to assess the intracellular ROS level. HT29 cells were seeded into a 24-well plate and cultured for 24 h. And then, the cells were treated with SASP or Ce-SASP-RS ICPs (200 μg/mL), with or without H2O2 (0.6 mM), for another 4 h. Furthermore, DCFH-DA was added into the culture medium for 20 min of co-incubation at 37 ℃. Finally, the cells were washed three times with PBS and measured through inverted fluorescence microscope.
Measurement of antibacterial efficacy in vitro
Escherichia coli (E. coli), strain ATCC25922 was selected for assessing the antibacterial activity of samples through the method of plate counting. To be specific, the bacteria solution was mixed with sterile solution containing Ce-SASP-RS ICPs or SASP through gentle shaking at 37 ℃. Then, the mixture was incubated in TSB culture medium at 37 ℃ for 24 h. After that, the solution of bacteria was diluted by PBS, and the dilutions with different concentrations were added onto dishes containing the nutrient agar. After another 16 h of incubation under similar conditions, the number of the colonies was counted. The antibacterial effect was calculated according to the following formula:
$$\text{R }(\text{\%})\hspace{0.17em}=\hspace{0.17em}(\text{B}-\text{C})\hspace{0.17em}\times \hspace{0.17em}100,$$
where R is antibacterial effect (%), B is the mean number of colonies in the control group, and C is the mean number of colonies in Ce-SASP-RS group or SASP group.
In vivo animal studies
All animal experiments have been approved by the Animal Care and Utilization Committee of Henan University of Science and Technology. C57BL/6 mice (8 weeks old, approximately 25 g, male) purchased from Liaoning Changsheng Biotechnology Co., Ltd. were used in this study.
DSS-induced model of colitis and treatment
After cohoused for 1 week, the mice were randomly divided into 4 groups (n = 6) as follows: PBS + Water, PBS + 5% DSS, Ce-SASP-RS ICPs + 5% DSS, SASP + 5% DSS. The healthy control group were fed with plain drinking water, and the other three groups were fed with 5% DSS (w/v, 36,000–50000 Da, MP Biomedicals) supplemented in drinking water for 6 consecutive days. Given that Ce-SASP-RS ICPs were unstable in stomach conditions, mice treated with DSS were respectively given PBS, Ce-SASP-RS ICPs (50 mg/kg) and SASP (50 mg/kg) through daily intraperitoneal injection. During the 6-day experimental period, the changes in body weight, stool consistency and fecal bleeding were recorded every day. And disease activity index (DAI) was calculated based on the above recorded changes. On the last day of the experiment, all the mice were euthanized, and the entire colon, main organs and intestinal contents were collected for analysis.
Histological analysis
The harvested colonic tissues were fixed in 4% formalin solution for 48 h. Subsequently, the samples were treated by ethanol solutions and xylene, followed by embedding in paraffin. After that, the samples were cut into sections with thick of 5 μm and mounted on slides for Hematoxylin–Eosin (H&E) staining. In addition, the histological analysis for main organs, including heart, liver, lung, spleen and kidney was performed with the same method.
ELISA analysis
The middle colon eviscerated from different mice was weighed and homogenized in saline at 4 ℃. And then, the obtained homogenate was centrifuged at 2000 rpm for 20 min at 4 ℃. After that, the supernatant was collected for detecting anti-inflammatory (IL-10) and pro-inflammatory cytokines (TNF-α, IL-1β) by commercial mouse IL-10, TNF-α and IL-1β ELISA Kit (Solarbio, China).
In vivo ROS analysis
The middle colon eviscerated from different mice was weighed and homogenized in saline containing TA (•OH indicatior) at 4 ℃. After that, the obtained homogenate was centrifuged at 2000 rpm for 20 min at 4 ℃. Subsequently, the supernatant was collected and detected by fluorescence spectrophotometer with excitation wavelength at 325 nm.
Gut microbiota analysis
Feces from each mouse were collected and frozen at -80 ℃ for bacterial diversity analysis by 16S gene sequencing technology. Briefly, microbiome DNA was extracted from colonic content by the hexadecyltrimethylammonium bromide (CTAB) method, and then sequenced by building a sequencing library on Illumina HiSeq. The results were stored in FASTQ (referred to as fq) format file, and data analysis was conducted using Qiime v.1.9.1 and LEfSe analysis package.
Statistical analysis
The results are shown as the mean ± standard deviation of the mean. The drawing software is GraphPad Prism and Origin. Student’s t test and one-way analysis of variance (ANOVA) were used in statistical significance analysis. Values with p
Results and discussion
Synthesis and characterizations of Ce-SASP-RS ICPs
Ce-SASP-RS ICPs were synthesized based on a one-step assembly of phenolic group-metal ion coordination complexes (Fig. 1a). Meanwhile, resistant starch was selected to modify Ce-SASP ICPs for regulating the intestinal flora disorder [32]. In detail, the phenolic carboxyl group and hydroxyl group in SASP are deprotonated by treating with NaOH solution. And the deprotonated SASP are subsequently coordinated with the metal ions Ce and further self-assemble to form Ce-SASP ICPs. Meanwhile, soluble starch is dissolved into boiling water and subjected to heat-hold-cool-hold temperature cycle to increase the resistant starch content [39]. Finally, resistant starch is grafted on the surface of Ce-SASP ICPs through hydrogen bond to construct Ce-SASP-RS ICPs. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Fig. 1c,e and Figure S1,S2) show that the as-prepared Ce-SASP-RS ICPs are irregular spherical particles with an average diameter of 24.7 ± 5.7 nm. And the SEM image as well as TEM image of Ce-SASP ICPs prepared in the absence of resistant starch (Fig. 1b,d) demonstrate that the resistant starch has negligible influence on the morphological attributes of Ce-SASP ICPs. The particle size distribution of Ce-SASP-RS ICPs was characterized by dynamic light scattering (DLS), and Ce-SASP-RS ICPs exhibit the average particle size of 61.74 nm with a narrow size distribution (Figure S3). The particle size determined by DLS is larger than that determined by SEM, which may be caused by the hydration layer in DLS measurement and trend of Ce-SASP-RS ICPs to aggregate into clusters. The energy dispersive spectroscopy (EDS) spectra (Figure S4) show that there are signals of C, O, Ce, N and S in Ce-SASP-RS ICPs. And the EDS elemental mapping (Fig. 1f) further reveals the uniform distribution of C, O, Ce, N and S elements in the prepared samples. The powder X-ray diffraction (PXRD) patterns of the samples (Fig. 1i) show that SASP has crystalline forms with typical characteristic peaks. While there are no obvious diffraction peaks in PXRD patterns of both Ce-SASP ICPs and Ce-SASP-RS ICPs, indicated the amorphization of SASP after coordinating with Ce3+. Fourier transform infrared (FTIR) analysis (Fig. 1j) shows that SASP has a typical peak at 1677 cm−1 assigned to the ν(C=O) vibration of the carboxylic group [40]. However, this peak is absent in the spectra of Ce-SASP ICPs, and new peaks at 1597 cm−1 and 1433 cm−1 assigned to the asymmetric stretching vibration ν(COO–) of the carboxylate group can be observed from the coordination polymers, indicating the coordination between Ce ions and carboxyl groups in SASP [41]. In addition, the stretching vibration band at 1281 cm−1 attributed to ν(C-O) of phenolic group in SASP is shifted to 1265 cm−1 after coordinating with Ce ions, indicated that the phenolic hydroxyl group of SASP also participate in the coordination process [41]. Meanwhile, the shift of the band assigned to δ(OH) in-plane bending from 1393 cm−1 in free SASP ligand to 1388 cm−1 in the coordination polymers further confirm the participation of phenolic group in the coordination process [41]. Furthermore, the peak at 529 cm−1 assigned to ν(Ce–O) stretching vibrations is observed in the spectrum of Ce-SASP ICPs[41]. Based on the comprehensive analysis of the FTIR results, it can be concluded that SASP act as a bidentate monoanionic chelating agent to coordinate with Ce ions via carboxylic and phenolic groups. And compared with the FTIR spectra of Ce-SASP ICPs, the characteristic peaks of soluble starch at 2927 cm−1, 1020 cm−1 and 1156 cm−1 are exhibited in the spectra of Ce-SASP-RS ICPs [42], indicated the successful grafting of soluble starch on the surface of Ce-SASP ICPs. 1H and 13C solid-state NMR experiments were performed to further evaluate the intermolecular interactions. The 13C NMR spectra (Figure S5a) show that characteristic resonance peaks of SASP are downfield shifted after coordinating with Ce ions. Meanwhile, the main proton peak in the 1H NMR spectrum of Ce-SASP-RS ICPs is downfield from 11.0 ppm to 10.07 ppm compared with that of free SASP (Figure S5b). These obvious changes of 1H and 13C signals in Ce-SASP-RS ICPs from the individual SASP indicate the existence of certain intermolecular interactions between Ce ions and SASP [43]. The results of zeta potential (Fig. 1g) show that the value changes from -38.2 ± 0.36 mV for SASP to -9.76 ± 0.31 mV for Ce-SASP ICPs, indicating the successful coordination between Ce ions and SASP. And the value further decreases to -4.75 ± 0.12 mV after grafting resistant starch on the surface of Ce-SASP ICPs. Though the zeta potential of SASP increased after interacting with Ce ions and soluble starch, Ce-SASP-RS ICPs still retain negative charge to facilitate the targeting of positively charged colonic lesions via electrostatic interactions [44].
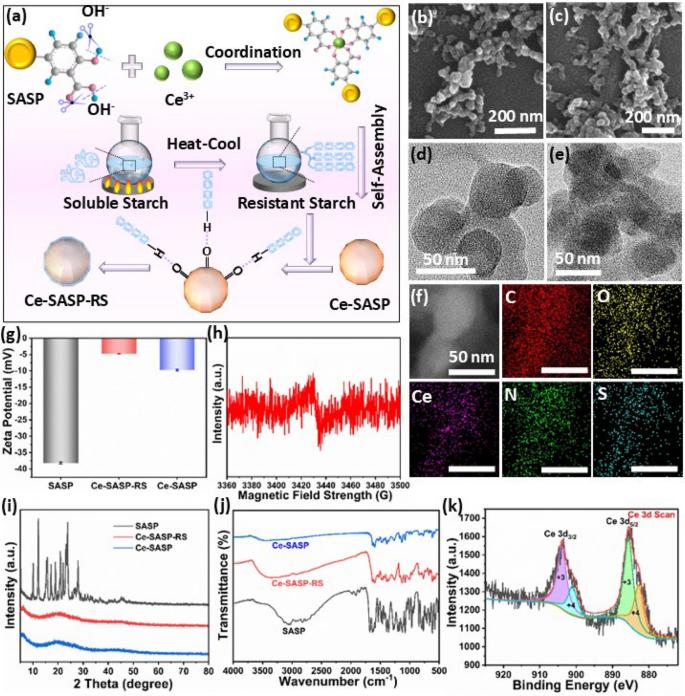
a The scheme of synthetic procedure of Ce-SASP-RS ICPs. SEM images of b Ce-SASP ICPs and c Ce-SASP-RS ICPs. TEM images of d Ce-SASP ICPs and e Ce-SASP-RS ICPs. f STEM elemental mapping of C, O, Ce, N and S elements in Ce-SASP-RS ICPs. g Zeta potential of Ce-SASP-RS ICPs, Ce-SASP ICPs and SASP. h EPR spectra of Ce-SASP-RS ICPs. i PXRD spectra of Ce-SASP-RS ICPs, Ce-SASP ICPs and SASP. j FTIR spectra of Ce-SASP-RS ICPs, Ce-SASP ICPs and SASP. k High resolution XPS spectra of Ce 3d in Ce-SASP-RS ICPs
X-ray photoelectron spectroscopy (XPS) analysis was performed to investigate the surface chemistry of Ce-SASP-RS ICPs (Figure S6). Figure 1k shows that the high-resolution XPS spectrum of Ce 3d can be resolved into four peaks. Specifically, the peaks with binding energies at 886.2 and 904.6 eV can be assigned to Ce3+, and the peaks with binding energies at 882.8 and 901.0 eV can be assigned to Ce4+, indicated the concurrence of Ce3+ and Ce4+ in Ce-SASP-RS ICPs. According to the reports, compounds containing Ce ions usually exhibit multiple enzyme activities, and the high ratio of Ce3+ to Ce4+ on their surfaces can guarantee the compounds good POD like activity, SOD like activity and •OH elimination ability, while restrained CAT-like activity [45]. Since excess SASP may oxidize Ce3+ into Ce4+ by phenolic carboxyl group, precisely controlling the molar ratio of SASP to Ce3+ is necessary during the preparation process. The proportion of Ce species in Ce-SASP-RS ICPs which are synthesized with different molar ratio of SASP/Ce are calculated based on the ratio of the comprehensive peak area of Ce3+ to the total peak area of Ce. The results (Figure S7 and Table S1) show that the proportion of Ce3+ to the total amount of Ce ions in Ce-SASP-RS ICPs synthesized with the molar ratio of SASP/Ce as 0.8 is about 77%. Though this value is slightly lower than that of Ce-SASP-RS ICPs synthesized with less SASP participated, it is still much higher than that of the reported CeO2 particles [23, 46]. As high loading amount of SASP in Ce-SASP-RS ICPs is also required for treating IBD, Ce-SASP-RS ICPs synthesized with molar ratio of SASP/Ce3+ as 0.8 is selected for the following experiments. And the analysis of inductively coupled plasma-optical emission spectrometry (ICP-OES) showed that the loading amounts of Ce ions in Ce-SASP-RS ICPs synthesized with this ratio is about 12.69% (w/w). Given that the coexistence of Ce3+ and Ce4+ may cause the emergence of oxygen vacancies in the complexes to balance the local charge [47], electron paramagnetic resonance (EPR) analysis was employed to characterize oxygen vacancies in Ce-SASP-RS ICPs. As confirmed in Fig. 1h, Ce-SASP-RS ICPs demonstrated an oxygen vacancy-related signal at approximately g = 2.0. The existence of oxygen vacancies is beneficial for Ce-SASP-RS ICPs to keep multiple enzyme-like catalytic activities because they can promote Ce4+ which are generated in the cascade reactions to recover to Ce3+ [24, 50].
ROS scavenging activity of Ce-SASP-RS ICPs
From the above results, it can be seen that the high ratio of Ce3+/Ce4+ and the presence of oxygen vacancies in Ce-SASP-RS ICPs would contribute to their POD-like activity, SOD-like activity and •OH elimination ability, whereas restrained CAT-like activity [23, 48]. That is to say Ce-SASP-RS ICPs not only can generate H2O2 and •OH to kill bacteria, but also can scavenge •O2− and •OH to defend the oxidative stress and maintain the redox balance through cascade reactions. Specifically, •O2− can be disproportionated to generate H2O2 catalyzed by Ce-SASP-RS ICPs owing to their SOD-like activity (Fig. 2a). Subsequently, the produced H2O2 can enter into the Fenton-like reactions to generate highly toxic •OH mediated by the POD-like activity of Ce-SASP-RS ICPs. Finally, the generated •OH can be downstream eliminated by Ce-SASP-RS ICPs due to the redox cyclability from Ce4+ to Ce3+ and the existence of oxygen vacancies. Since excessive oxidative stress and dysbiosis of the gut microbiota are closely related with the pathogenesis of IBD [2], the ability of Ce-SASP-RS ICPs for simultaneously generate H2O2 and •OH to kill bacteria and scavenge •O2− and •OH to defend the oxidative stress, can be rightly applied for IBD management. To further confirm this hypothesis, the multi-enzymatic activities of Ce-SASP-RS ICPs, including SOD-like activity, POD-like activity and •OH elimination ability were evaluated with reported methods.
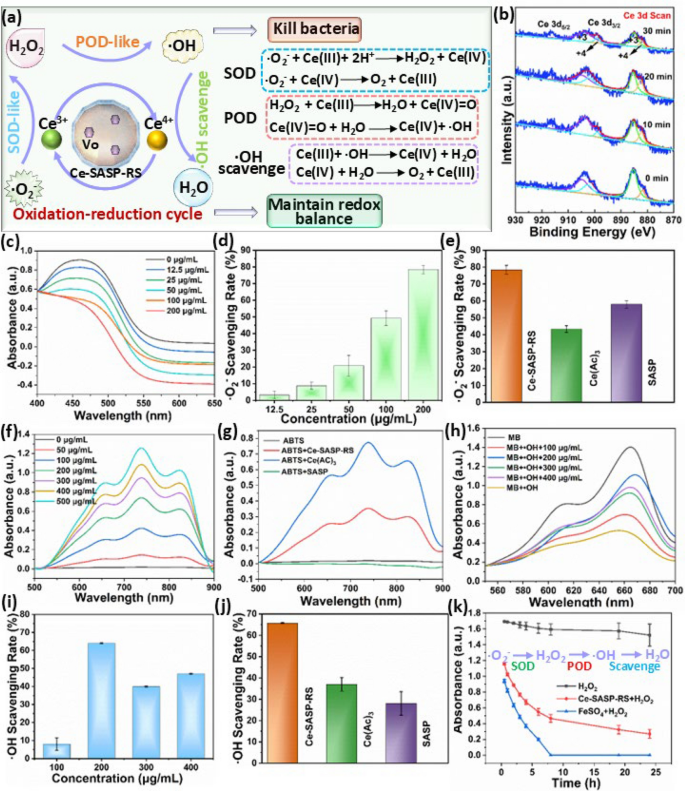
a Schematic illustrating the mechanism of multienzyme mimicking activities of Ce-SASP-RS ICPs for simultaneously generate H2O2 and •OH to kill bacteria, and scavenge •O2− and •OH to maintain redox balance. b High-resolution XPS spectra of Ce 3d in Ce-SASP-RS ICPs after interacting with H2O2 for different times. c The UV–vis absorption spectra of oxWST-8 and d the corresponding •O2− scavenging rate after the reaction through the catalysis of Ce-SASP-RS ICPs with different concentrations. e •O2− scavenging rate of Ce-SASP-RS ICPs, Ce(Ac)3 and SASP under the same experimental conditions. f The UV–vis absorption spectra of ABTS•+ after the catalytic effect of Ce-SASP-RS ICPs with different concentrations for 30 min. g The UV–vis absorption spectra of ABTS•+ after the catalytic effect of Ce-SASP-RS ICPs, Ce(Ac)3 and SASP under the same experimental conditions. h The UV–vis absorption spectra of MB in the presence of •OH and i the corresponding •OH scavenging rate after the catalytic effect of Ce-SASP-RS ICPs with different concentrations. j •OH scavenging rate of Ce-SASP-RS ICPs, Ce(Ac)3 and SASP under the same experimental conditions. k The UV–vis absorption intensity of MB respectively treated by H2O2, Ce-SASP-RS + H2O2 or FeSO4 + H2O2 for different times
Initially, the ability of reversible conversion between Ce3+ and Ce4+ in Ce-SASP-RS ICPs during the process of scavenging ROS was investigated to ensure their multi-enzymatic activities. The EPR analysis (Figure S8) shows that the oxygen vacancy related signal (g ≈ 2.0) in Ce-SASP-RS ICPs is obviously enhanced after interacting with H2O2, indicated the change of valence state of Ce ions. To further evaluate the amplitude of variation of Ce3+, XPS analysis was performed on Ce-SASP-RS ICPs which were reacted with H2O2 solution for different times. The results (Fig. 2b and Table S2) show that the Ce3+ amount in Ce-SASP-RS ICPs fluctuate within the range of 64% ~ 83% accompanied by an extension of reaction time, indicated a continuous valence state transition between Ce3+ and Ce4+ during this process. The above results suggested that Ce-SASP-RS ICPs can exhibit multi-enzymatic activities sustainably.
Encouraged by this conclusion, the SOD-like activity of Ce-SASP-RS ICPs for •O2− elimination was firstly investigated with WST-8 assay kit which can be reduced to WST-8 formazan by •O2−. In detail, •O2− generated via the xanthine/xanthine oxidase system reduced WST-8 to WST-8 formazan, with a typical absorption peak at 450 nm. However, the absorption intensity of WST-8 formazan obviously declines with Ce-SASP-RS ICPs added into the above system (Fig. 2c), which is ascribed to the SOD-like activity of Ce-SASP-RS ICPs to eliminate •O2−. In addition, the •O2− elimination efficiency of Ce-SASP-RS ICPs is positively correlated with particle concentration, and •O2− elimination level can reach over 70% with a Ce-SASP-RS ICPs concentration of 200 μg/mL (Fig. 2d), demonstrating the good SOD-like activity of Ce-SASP-RS ICPs. Meanwhile, both free Ce(AC)3 and free SASP exhibited weaker •O2− elimination efficiencies as compared with the same concentration of Ce-SASP-RS ICPs (Fig. 2e). Subsequently, the POD-like activity of Ce-SASP-RS ICPs was investigated by employing 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate) (ABTS) as indicator. Based on the mechanism that POD-like nanozymes can catalyze H2O2 to oxidize ABTS into blue product ABTS•+, Ce-SASP-RS ICPs were mixed with solution containing ABTS and H2O2, followed by the UV–vis analysis of absorption peak at 740 nm. Figure 2f shows that the absorbance intensity of ABTS•+ in the mixture solution of ABTS and H2O2 is significantly improved after the additon of Ce-SASP-RS ICPs, indicating that Ce-SASP-RS ICPs can act as POD-like nanozymes to increase the electron transfer between ABTS and H2O2 [49]. And the POD-like activity of Ce-SASP-RS ICPs is concentration-dependent and positively correlated with time (Fig. 2f and Figure S9). In addition, the POD-like activity of free SASP and free Ce(AC)3 were also evaluated with the same method. The result (Fig. 2g) shows that SASP has negligible SOD-like activity. Conversely, Ce(AC)3 can make more ABTS•+ generated in the mixture solution of ABTS and H2O2 compared to that of Ce-SASP-RS ICPs with the same concentration. Combined with the result that Ce-SASP-RS ICPs have higher SOD-like activity than that of free Ce(Ac)3 (Fig. 2e), it can be concluded that the coordination with SASP can make Ce3+ ions caused SOD-like activity enhanced while the POD-like activity decreased. This property can guarantee Ce-SASP-RS ICPs to accelerate the •O2− scavenging rate whereas slow down the •OH generation rate, which is beneficial for Ce-SASP-RS ICPs to defend oxidative stress while using free radicals to kill bacteria. Furthermore, •OH elimination ability of Ce-SASP-RS ICPs was investigated through colorimetric assay by using methylene blue (MB) as •OH indicator. In the classic Fe2+/H2O2 Fenton system for producing •OH [50], MB is oxidized and discolored by •OH with its typical absorption peak intensity at 664 nm decreased. Whereas the discoloration of MB is obviously inhibited with the addition of Ce-SASP-RS ICPs into the system, indicating that Ce-SASP-RS ICPs have ability to scavenge •OH (Fig. 2h). And the influence of Ce-SASP-RS ICPs amount on their •OH scavenging efficiency was also evaluated by using different concentration gradients of Ce-SASP-RS ICPs to scavenge •OH in the experiments. The results (Fig. 2h,i) show that the particle concentration of 200 μg/mL has high •OH scavenging efficiency of over 65%. However, the corresponding value is obviously declined as the particle concentration used exceeds 200 μg/mL. This phenomenon may be caused by the competition between •OH generation and elimination related to Ce-SASP-RS ICPs. In addition, •OH scavenging activity of SASP (200 μg/mL) and Ce(Ac)3 (200 μg/mL) are calculated to be 28% and 37%, respectively (Fig. 2j), which are much lower than that of Ce-SASP-RS ICPs with the same concentration. This result indicates that the •OH scavenging ability based on Ce ions can be improved by coordinating with SASP. On the other hand, dissolved oxygen contents in distilled water containing H2O2 were detected by dissolved oxygen analyzer to evaluate the catalase (CAT)-like activity of Ce-SASP-RS ICPs. Figure S10 shows that the increase of O2 concentration in distilled water containing H2O2 is obviously reduced after the addition of Ce-SASP-RS ICPs, indicated the absence of catalase (CAT)-like activity of Ce-SASP-RS ICPs to decompose H2O2 into O2 and H2O. Meanwhile, POD-like activity of Ce-SASP-RS ICPs can consume H2O2 to generate •OH, inducing the lower increase of O2 content compared to distilled water containing H2O2. This result suggests that Ce-SASP-RS ICPs mainly catalysis H2O2 into •OH instead of O2 and H2O. To further confirm the cascade reactions caused by Ce-SASP-RS ICPs in the presence of H2O2, the discolourization rate of mixture solution containing MB and H2O2 was monitored after addition of Ce-SASP-RS ICPs or FeSO4. Figure 2k shows that the absorbance intensity of the mixture solution declined with Ce-SASP-RS ICPs added, indicated the generation of •OH caused by the POD-like activity of Ce-SASP-RS ICPs. Moreover, the discolourization rate of MB in the mixture solution caused by FeSO4 is much higher than that caused by Ce-SASP-RS ICPs. As shown in Fig. 2k, MB in the mixture solution almost completely degrade after incubating with FeSO4 for 8 h. However, after the same processing time, the discolourization rate of MB in the mixture solution incubated with Ce-SASP-RS ICPs is about 78%, and even after 24 h of treatment, the MB discolourization rate still doesn’t go to zero (82.3%). This phenomenon may be caused by •OH scavenging ability of Ce-SASP-RS ICPs. Collectively, it can be seen that Ce-SASP-RS ICPs really participated in the cascade reactions of generating H2O2 owing to the SOD-like activity, producing •OH due to POD-like activity, and finally scavenging •OH. As it will take some time for Ce-SASP-RS ICPs to eliminate •OH, the generated •OH have opportunity to kill bacteria. Due to the reversible conversion between Ce3+ and Ce4+, this cascade reactions can achieve dynamic equilibrium for ROS generation and elimination. Thus, large amount of •OH can be generated to kill bacteria and finally be eliminated completely, which make Ce-SASP-RS ICPs possible to maintain redox balance while killing bacteria.
Antibacterial capability of Ce-SASP-RS ICPs with maintaining redox balance in vitro
To further prove the antibacterial capability of Ce-SASP-RS ICPs with maintaining redox balance, the antibacterial function of Ce-SASP-RS ICPs was firstly evaluated through the method of plate counting with using Escherichia coli (E. coli) as representative bacteria. After treated with PBS (control), Ce-SASP-RS ICPs or free SASP, the bacterial colonies were photographed and the colony forming units (CFUs) were measured to calculate antibacterial rate. Compared with the control group, the drug SASP shows an antibacterial rate of 65% at the dosage of 200 μg/mL (Fig. 3a–c and Figure S11). Moreover, the antibacterial rate of Ce-SASP-RS ICPs can even increase to 90% with using the same dosage (Fig. 3a–c and Figure S11), indicating that the antibacterial efficiency of SASP can be increased by coordinating with Ce3+. Given that •OH can combat bacterial infection efficiently [51], the increase of antibacterial rate of SASP after coordinating with Ce3+ can be ascribed to their new gained POD-like activity during the formation of Ce-SASP-RS ICPs.
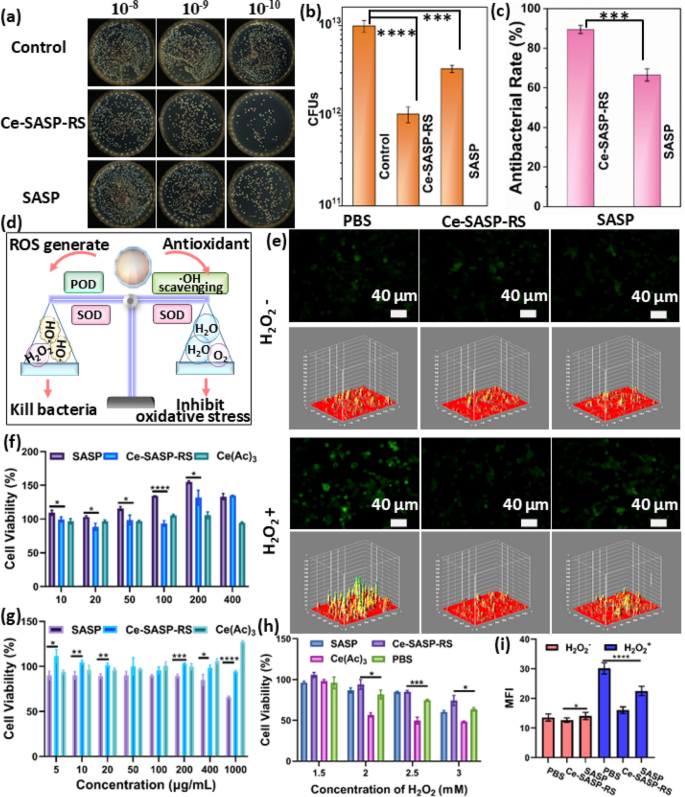
a Images of bacteria colonies formed by E. coli with treatment of PBS, Ce-SASP-RS ICPs or SASP. b The corresponding CFU counts of E. coli. with various treatments. c The corresponding antibacterial rate with various treatments. d Schematic illustrating the capability of Ce-SASP-RS ICPs to kill bacteria by ROS and simultaneously maintain redox balance. e Fluorescence images and i the corresponding fluorescence intensity of DCFH-DA in H2O2 treated HT29 cells after incubated with Ce-SASP-RS or SASP. Cell viability of f RAW 264.7 cells and g HT29 cells treated with different concentrations of Ce-SASP-RS ICPs, SASP or Ce(Ac)3 for 24 h. h Cell viability of H2O2 (1.5, 2, 2.5 and 3 mM) treated RAW264.7 cells after incubated with Ce-SASP-RS, SASP or Ce(Ac)3 (200 μg/mL). Data were expressed as the mean ± SD (n = 3). Statistical analysis was performed using one-way analysis of variance (ANOVA) analysis and Student’s t test. *P **P ***P ****P
Subsequently, the ability of Ce-SASP-RS ICPs to maintain redox balance was evaluated in cancer cells. Since cell viability is closed related to intracellular ROS level [52], the influence of Ce-SASP-RS ICPs on the survival rate of Raw 264.7 macrophages and human colon epithelial cells (HT29) was evaluated through CCK-8 method (Fig. 3f, g). Unlike the result that Ce-SASP-RS ICPs can kill bacteria by generating •OH, Ce-SASP-RS ICPs show negligible cytotoxicity towards the two cell lines even up to 400 μg/mL. This distinction may be caused by the different sensitivity of cancer cells and bacteria to the enhanced ROS levels [52]. In other words, the Ce-SASP-RS ICPs caused increase of ROS levels in bacterial cells is harmful to bacterial cells, but the increased ROS level in cancer cells is still safe for cancer cells. In addition, it can be found that with the concentration of SASP and Ce-SASP-RS ICPs increased, the cell viability of RAW 264.7 cells was increased, while the cell viability of HT29 cells was decreased. This result may be caused by the different mechanisms of drug action of SASP in RAW 264.7 cells and HT29 cells. As RAW 264.7 cells are macrophages that are sensitive to inflammation, their survival rate can be increased by SASP due to its anti-inflammatory activity [53, 54]. On the other hand, HT29 cells are colorectal cancer cells which can be killed by SASP through inhibition of plasma membrane cystine transporter xc − [55]. To further investigate the multi-enzymatic activities of Ce-SASP-RS ICPs for simultaneously generating and eliminating ROS, the cell models with different intracellular ROS levels were established by incubating cells with different concentrations of H2O2. Due to the oxidative stress caused by H2O2, the cell viability of Raw 264.7 macrophages is dose dependent on H2O2 within 1.5 to 3 mM (Fig. 3h). And co-incubation of Ce-SASP-RS ICPs can relieve this oxidative stress as the corresponding cell viability was recovered to almost 100% under the condition that the used concentration of H2O2 is less than 2 mM. However, with the concentration of H2O2 continued to increase to 3 mM, the survival rates of cells can only recover from 63% to 73% after the treatment of Ce-SASP-RS ICPs. This phenomenon may be caused by the competition between enzyme activity of Ce-SASP-RS ICPs to catalyze H2O2 to generate •OH for killing cells and to scavenge •OH to inhibit oxidative stress in cancer cells (Fig. 3d). The •OH scavenging ability of Ce-SASP-RS ICPs play the dominant role with the concentration of H2O2 within 2 mM, while •OH generation efficiency may surpass •OH elimination efficiency with the concentration of H2O2 higher than 2 mM. It can be concluded that Ce-SASP-RS ICPs can maintain redox balance in cancer cells within a certain degree of oxidative stress. In addition, the drug SASP can weakly protect cells from H2O2 because of their low SOD-like activity, low •OH scavenging ability and negligible POD-like activity compared to that of Ce-SASP-RS ICPs. Moreover, the treatment of Ce(Ac)3 can even exacerbate the cytotoxicity of H2O2 to cancer cells once the concentration of H2O2 exceeded 1.5 mM. This result can be ascribed to its higher POD-like activity to generate •OH and lower •OH scavenging activity to reduce oxidative stress compared to that of Ce-SASP-RS ICPs under the same conditions. To further evaluate the ability of Ce-SASP-RS ICPs for maintaining redox balance in cancer cells within a certain degree of oxidative stress, the intracellular ROS levels were investigated by using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as ROS indicator, which can form green fluorescent DCF by interacting with intracellular ROS (Fig. 3e and Figure S12). Compared with untreated HT29 cells or cells only treated with Ce-SASP-RS ICPs (control groups with mean fluorescence intensity (MFI) = 13.58 or 12.67), green fluorescence signal in cells treated with H2O2 (0.6 mM) show significant increase (MFI = 30.15) due to the formed oxidative stress conditions (Fig. 3i). However, this increased DCF fluorescence signal can be remarkably reduced to the level (MFI = 16.05) similar to that of control group after the treatment of Ce-SASP-RS ICPs, indicating that Ce-SASP-RS ICPs can maintain intracellular redox balance within a certain concentration of H2O2. And due to the weak ROS scavenging ability of SASP, the H2O2 induced DCF fluorescence signal intensity can only be reduced to 22.48 after the treatment of SASP. Therefore, by using Ce ions to coordinate with SASP, the ROS scavenging ability of SASP can be greatly enhanced, so as to increase the cell protective effect. Furthermore, the influence of Ce-SASP-RS ICPs on cells caused by the ability of both generating and scavenging ROS was explored through measuring the levels of DNA oxidative damage biomarker (8-hydroxy-20-deoxyguanosine, 8-OHdG), pro-inflammatory cytokine IL-1β and anti-inflammatory IL-10 in RAW 264.7 cells. As shown in Figure S13, the content of 8-OHdG, IL-1β and IL-10 in cells treated with Ce-SASP-RS ICPs is similar to that in cells treated with PBS. This result suggests that Ce-SASP-RS ICPs can’t cause redox imbalance in cells because the over produced ROS by Ce-SASP-RS ICPs can be eliminated by themselves through cascade reactions. Due to the oxidative stress caused by H2O2, the content of 8-OHdG and IL-1β in cells have significant increase, and the content of IL-10 in cells obviously decrease after treated with H2O2. However, the treatment of Ce-SASP-RS ICPs can alleviate the terrible oxidative stress situation by diminishing the level of 8-OHdG and IL-1β, and increasing the level of IL-10 in cells. This result further reveals that Ce-SASP-RS ICPs can modulate redox balance in cells.
In vivo therapeutic efficacy of Ce-SASP-RS ICPs in DSS-induced colitis mice
Encouraged by the excellent antibacterial capability of Ce-SASP-RS ICPs with maintaining redox balance in vitro, the potential application of Ce-SASP-RS ICPs for treating colitis was further investigated in vivo. Firstly, the stability of Ce-SASP-RS ICPs in solution with pH values mimicking the environments of stomach or gut was evaluated for determining the method of administration. Figure S14 shows that the cumulative release rate of Ce ions from Ce-ICP-RS ICPs reaches over 80% after incubating in buffer solution with pH = 1.2 for 24 h. On the other hand, there are almost no Ce ions released from Ce-SASP-RS ICPs after dispersing in buffer solution with pH = 4.0, 7.4, and 8.0. In view of pH values in stomach (~ pH 1.2) and colon (~ pH 7.4) [56], it can be concluded that Ce-SASP-RS ICPs are quite stable in colon, but are easily decomposed in stomach. Therefore, intraperitoneal injection was selected as the administration mode for subsequent animal experiments to achieve more ideal effects of the drug. To further prove that Ce-SASP-RS ICPs can be absorbed into the colon tissues through this administration mode, PBS or PBS containing Ce-SAP-RS ICPs (50 mg/kg) were intraperitoneally injected into C57BL/6 mice (once a day, 6 consecutive days), and colon tissues were collected for analysis with inductively coupled plasma emission spectrometer (ICP-OES). The results (Figure S15) show that the content of Ce ions is about 25.84 ± 0.27 μg/g(tissues) in colon tissues from mice treated with Ce-SASP-RS ICPs, which is much higher than that treated with PBS (0.48 ± 0.15 μg/g(tissues)). This result indicates that intraperitoneal injection is a feasible administration mode for Ce-SASP-RS ICPs to treat IBD.
The therapeutic effect of Ce-SASP-RS ICPs in vivo was evaluated on DSS induced colitis mouse (C57BL/6) model. As shown in Fig. 4a, mice were given drinking water containing 5% (w/v) DSS for 6 consecutive days to cause colitis. Meanwhile, mice were randomly divided into three groups (n = 6) and respectively treated with PBS, Ce-SASP-RS ICPs and SASP through daily intraperitoneal injections. Unlike the gradually increased weight of healthy mice, DSS-induced colitis mice show a significant body weight loss and decreased by about 20% within 6 days, compared with the initial weight. And the weight loss of colitis mice treated with SASP was also very serious. Fortunately, Ce-SASP-RS ICPs can alleviate the weight loss of colitis mice with weight decrease by about 10% after 6 days of treatment (Fig. 4b). In addition, DSS-induced colitis mice had symptoms of diarrhea and bloody stools (Fig. 4f). And SASP treated colitis mice are in the same way. However, Ce-SASP-RS ICPs significantly inhibit such symptoms. Moreover, the disease activity index (DAI) was calculated based on fecal characteristics, occult blood levels, and weight reduction. Figure 4e shows that DAI values in the DSS group (20.8) and SASP group (20.4) are much higher than that in control group (0) and Ce-SASP-RS group (9.6), indicating that Ce-SASP-RS ICPs can rescue DSS-induced colitis. As another parameter for evaluating colitis phenotype, the colon length of mice in different groups were measured. And the results (Fig. 4c,d) show that colon length in DSS-induced colitis mice are much lower than that in healthy mice. Whereas this DSS-induced shortening of colon length can be well protected by Ce-SASP-RS ICPs compared with that in SASP treated group. The histopathological changes of colon in mice with different treatment were investigated by staining colon tissues with hematoxylin and eosin (H&E). As shown in Fig. 4g, colons of mice treated by DSS have severe damage with thinning of mucous membranes, disappearance of intestinal gland structure and high level of inflammatory cell infiltration. Whereas the treatment of Ce-SASP-RS ICPs can effectively relief this damage as well as reducing colonic histological damage scores (Fig. 4h).
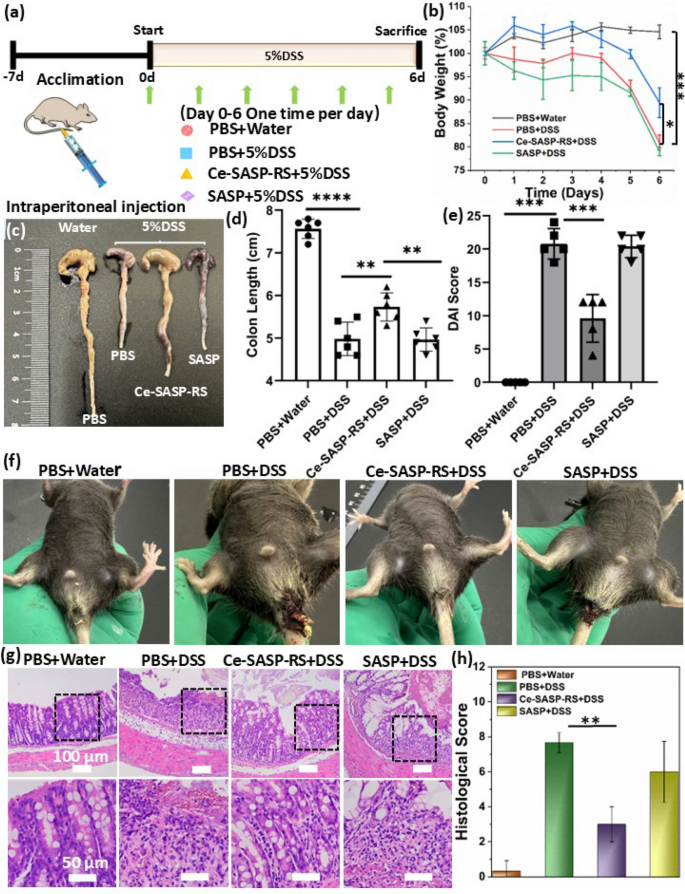
a Experimental timeline for treating DSS-induced colitis mice by Ce-SASP-RS ICPs. The mice were fed with water or water containing 5% DSS for 6 days. Meanwhile, PBS, Ce-SASP-RS ICPs or SASP was administrated to mice through intraperitoneal injection once a day. b Daily body weight changes of mice from groups with different treatments (n = 6). c Images of cecum-colon tissues and d colon length with different treatments (n = 6). e DAI scores for mice in different groups (n = 5). f Representative photographs of rectal areas of a healthy mouse and IBD mice after treatment with PBS, Ce-SASP-RS ICPs and SASP, respectively (on day 6). g Representative H&E staining images of colon tissues in mice with different treatments. h Colonic histological damage scores for mice in different groups (n = 3). Data were expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) analysis and Student’s t test. *P **P ***P ****P
To explore the ability of maintaining redox balance in intestinal tract by Ce-SASP-RS ICPs, the colon tissues were collected, ground and detected by terephthalic acid, a kind of ROS indicator which can emit fluorescence after interacting with •OH. Figure 5a and Figure S16 show that the colon tissues from DSS-induced colitis mice have significant fluorescence signal compared to that of healthy mice, while the fluorescence intensity can be slightly minimized by treating with SASP. Moreover, the treatment of Ce-SASP-RS ICPs can even reduce the fluorescence intensity to the level similar to that of healthy mice. These results suggested that the drug SASP are endowed with strong antioxidant effect by coordinating with Ce ions, inducing the intestinal redox level of colitis mice recovered to normal level. Furthermore, the microenvironment with restored redox balance facilitates Ce-SASP-RS ICPs to better play the role of anti-inflammation. To access the anti-inflammatory ability of Ce-SASP-RS ICPs in vivo, the essential inflammatory factors in colonic homogenates, including pro-inflammatory factors (TNF-α, IL-1β) and anti-inflammatory factors (IL-10) were detected by an ELISA assay. As shown in Fig. 5c–e, the treatment of Ce-SASP-RS ICPs can significantly reduce the levels of TNF-α and IL-1β while enhance the levels of IL-10, compared to that of DSS-induced colitis mice. In addition, drug SASP exhibits modest influence on the above inflammatory factors. These findings prove that Ce-SASP-RS ICPs have better ability to modulate the inflammatory microenvironment of DSS-induced colitis than that of free SASP, which may benefit from their ability of modulating redox balance in the intestine [18]. Though the broad-spectrum antibacterial effect of ROS may aggravate the dysbiosis of gut microbiota [57], superfluous •OH produced by Ce-SASP-RS ICPs can be eliminated by themselves to regulate redox balance, which provides a favorable environment for resistant starch to modulate the gut microbiota (Fig. 5b). And to evaluate the regulative effect of intestinal flora balance caused by Ce-SASP-RS ICPs, stool samples were analyzed by using advanced 16S rRNA sequencing technology. Figure 5f–h show that the bacterial richness (observed operational taxonomic units (OTUs) richness) and α-diversity of DSS-induced colitis mice are noticeably lower than that of healthy mice. And the treatment of free SASP has negligible effect to alleviate this undesirable phenomenon. Nevertheless, the corresponding values in Ce-SASP-RS ICPs treated mice have significant increase compared to that of DSS-induced colitis mice. Principal components analysis (PCA) was performed to investigate the β-diversity of intestinal microbiota. The PCA plots (Fig. 5i) demonstrate that DSS-induced colitis mice are significantly different from that of healthy mice. Whereas the gut microbiota profile of the Ce-SASP-RS ICPs treated group is closer to that of healthy mice, compared to other DSS-treated controls. The above results indicated that the intervention of Ce-SASP-RS ICPs can improve intestinal flora of DSS-induced colitis mice. Subsequently, an in-depth analysis was carried out to further investigate the gut microbiota. The relative abundance at genus level (Fig. 5j) show that the bacteria Prevotella associated with colitis decrease significantly in Ce-SASP-RS ICPs treated group (0.83%), compared to that in DSS-induced group (11.18%) (Figure S17a) [58]. Meanwhile, the relative abundance of beneficial bacteria, such as Bifidobacterium (maintain a proper health status by producing a number of potentially health promoting metabolites [59]) have an obvious growth in Ce-SASP-RS ICPs treated group (7.96%) compared to that in DSS-induced group (0.28%) (Figure S17b). The beneficial bacteria Allobaculum which can exert anti-inflammatory effects, protect intestinal barrier function and modulate human metabolism [60], is significantly reduced after DSS treatment (3.02%). However, the treatment of Ce-SASP-RS ICPs can make the recovery of this bacteria abundance (14.67%) and even surpass normal group (8.75%) (Figure S17c), because short chain fatty acids generated by resistant starch on the surface of Ce-SASP-RS ICPs are closely related to Allobaculum [61]. In addition, the beneficial bacteria CAG-485 which was related to host disease resistance [62], exhibited significant decrease in the DSS group (0.53%) (Figure S17d). The treatment of Ce-SASP-RS ICPs can make the bacteria abundance slightly increase (2.5%), indicated the positive therapy effect. However, the level of the bacteria abundance is still lower than that in the normal group (23.02%). This result may be caused by the short treatment period which can’t guarantee Ce-SASP-RS ICPs to cure IBD completely. Combining the antibacterial ability of Ce-SASP-RS ICPs mentioned in Fig. 3a–c with the result that Ce-SASP-RS ICPs treatment causing the suppression of harmful bacteria and the proliferation of beneficial bacteria, it can be concluded that Ce-SASP-RS ICPs can protect the intestinal microecological homeostasis while killing intestinal bacteria. In addition, Ce-SASP-RS ICPs treatment significantly decrease the relative abundance of Faecalibaculum (1.43%), a bacteria related to intestinal oxidation (negatively associated with SOD and GSH, and positively associated with ROS) [63], compared to that in DSS-induced colitis mice (7.24%), and is similar to that in healthy mice (1.83%) (Figure S17(e)). This result further indicates that Ce-SASP-RS ICPs can maintain intestinal redox balance owing to their multi-enzymatic activities. The heatmap (Figure S18) of the relative abundance of the top 50 OTUs at class level illustrates that the composition and function of the intestinal flora in the Ce-SASP-RS ICPs treatment group are similar to that in normal group. Moreover, the specialized microbial communities between Ce-SASP-RS ICPs treated group and DSS induced group was identified by the linear discriminant analysis (LDA) effect size (FEfSe) analysis. Figure S19 shows an obvious difference in gut microbiota between the above mentioned two groups. The harmful bacteria, including Prevotella and Faecalibaculum play a major role in the DSS induced group, while the probiotics, such as Allobaculum, Bifidobacteriaceae and Bifidobacterium are predominated in the Ce-SASP-RS ICPs treated group. The above results collectively indicate that Ce-SASP-RS ICPs can effectively regulate the intestinal symbiotic microbiota to a perfect state, which is of great significance for the treatment of IBD.
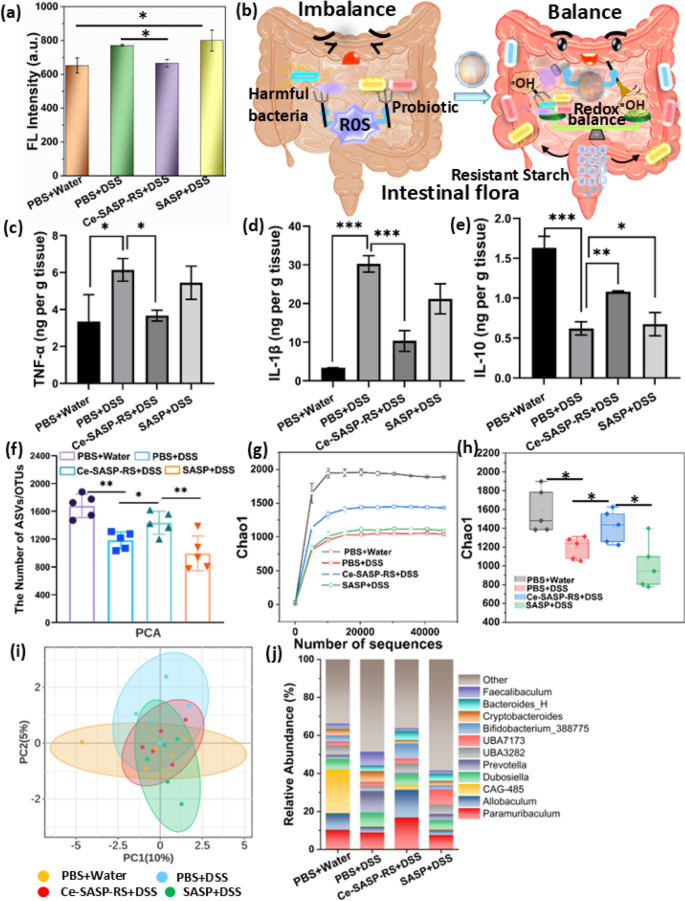
a ROS levels in colon tissue of different treatment groups measured by ROS fluorescence probe (n = 3). b Schematic illustrating the capability of Ce-SASP-RS ICPs to regulate the intestinal commensal microbiota. The levels of c TNF-α, d IL-1β, and e IL-10 cytokines in colon tissues from mice with different treatments (n = 3). f Analysis of microbial community observed OTU richness of gut microbiota in mice with different treatments (n = 5). Analysis of α-diversity of gut microbiota in mice with different treatments determined by g species rarefaction curve, and h grouping boxplot (n = 5). i Analysis of β-diversity of gut microbiota in mice with different treatments determined by principal component analysis (PCA) (n = 5). j The relative abundance histogram of gut microbiota at genus level (n = 5). Data were expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) analysis and Student’s t test. *P **P ***P ****P
Finally, the major organs including lung, heart, liver, spleen and kidney from mice with different treatments were analyzed with H&E staining to evaluate the in vivo toxicity of Ce-SASP-RS ICPs (Figure S20). Compared with the control group, there is no significant cell damage or pathological abnormalities in organs of mice treated by Ce-SASP-RS ICPs. This result indicates that Ce-SASP-RS ICPs can act as a safe bionanoplatform for the treatment of IBD.


