Preparation of CYM@MON-SA nanoparticles
The synthesis of CYM@MON-SA nanoparticles follows the procedure outlined in Fig. 1. MON-OH nanoparticles were initially synthesized in an aqueous solution using TEOS and BTSPD as silane precursors, CTAT as a surfactant template, and triethanolamine (TEA) as a catalyst (Fig. 1A). After removing the CTAT from the pores of MON-OH, amino groups were introduced by reaction with APTES, resulting in the formation of MON-NH2 nanoparticles (Fig. 1B). Subsequently, salicyloyl chloride was synthesized by chlorinating the carboxyl group of SA and reacted with the amino group of MON-NH₂ via nucleophilic substitution, yielding MON-SA nanoparticles (Fig. 1C). Finally, CYM was loaded onto the MON-SA nanoparticles using the solvent evaporation method, producing the functionalized CYM@MON-SA nanoparticles for further studies (Fig. 1D). Design rationale for this nanoplatform is illustrated in Fig. 1E, CYM@MON-SA is stimuli-responsive to plant-derived glutathione and amidase after foliar application, thereby enabling controlled release of biodegradable silicon, SA, and CYM. Several studies have demonstrated effective SA encapsulation in nanomaterials to enhance stability and bioactivity [4, 25, 35]. In this study, CYM@MON-SA co-delivering SA and CYM represent a novel nanopaticle-based fungicide for plant disease control.
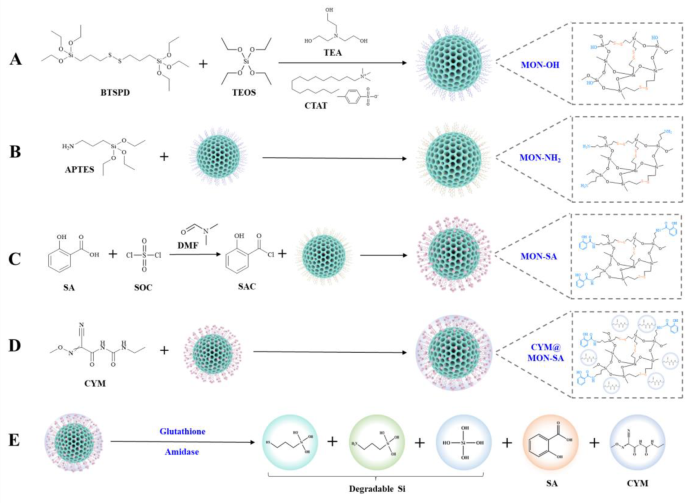
Schematic diagram of the synthesis pathway of CYM@MON-SA nanoparticles and its mechanism of responsive release. (A) MON-OH, (B) MON-NH2, (C) MON-SA, and (D) CYM@MON-SA synthesis pathways; (E) Responsive release mechanism of CYM@MON-SA triggered by glutathione (GSH) and amidase. BTSPD: bis-[3-(trimethoxysilyl)propyl]-disulfide; TEOS: tetraethyl orthosilicate ester; TEA: triethanolamine; CTAT: cetyltrimethylammonium ptoluenesulfonate; APTES: 3-aminopropyltriethoxysilane; SA: salicylic acid; SOC: thionyl chloride; SAC: salicyloyl chloride; DMF: N,N-dimethylformamide; CYM: cymoxanil
Characterization of CYM@MON-SA nanoparticles
To confirm the successful preparation of the nanoparticles, various characterization techniques were employed. As shown in Figs. 2A and B, the four types of nanoparticles (MON-OH, MON-NH2, MON-SA, and CYM@MON-SA) exhibited spherical morphology under TEM, with average particle sizes of 71.66, 74.95, 76.54 and 79.87 nm, respectively. The typical ordered mesoporous structures of MON-OH and MON-NH2 are clearly observed in the magnified TEM images. After grafting with SA and loading with CYM, the mesoporous channels were partially obscured by the organic compounds, and the surfaces of MON-SA and CYM@MON-SA became roughened. Additionally, elemental mapping under TEM (Fig. 2C) and EDS analysis (Fig. 2D) demonstrated a uniform distribution of carbon (C), nitrogen (N), oxygen (O), silicon (Si), and sulfur (S) throughout the CYM@MON-SA framework, confirming the successful incorporation of disulfide bonds and the effective functionalization with amino groups.
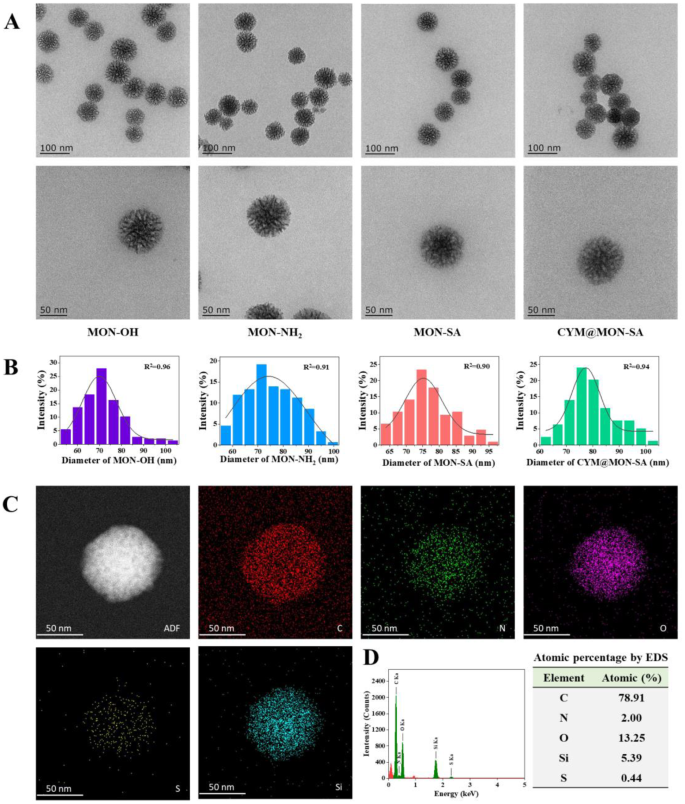
Morphology and element analysis of nanoparticles under transmission electron microscopy (TEM). (A) Morphological images; (B) Particle size distribution of four nanoparticles; (C) Elemental mapping; and (D) Energy-dispersive spectroscopy (EDS) analysis of CYM@MON-SA
Dynamic light scattering (DLS) results further confirmed functionalization, as the hydrodynamic diameter increased from 106.78 nm for MON-OH to 213.29 nm for CYM@MON-SA (Table 1). The hydrodynamic diameter is typically larger than the particle size observed under TEM, which can be attributed to hydration effects, surface modifications, aggregation phenomena, and the influence of water molecules and ions on the surface behavior of the particles [43]. Additionally, changes in zeta potential reflected the successful stepwise synthesis and functionalization of these four nanoparticles (Table 1). The initial zeta potential of MON-OH was measured at − 23.03 mV. After the introduction of positively charged amino groups, MON-NH₂ exhibited a drastic potential reversal to +25.41 mV [9, 44]. However, the MON-SA solution showed a negative potential of − 22.32 mV due to the successful grafting and dissociation of SA, which formed negatively charged salicylate ions in neutral or alkaline environments. Moreover, CYM@MON-SA maintained a negative potential of − 29.32 mV attributed to the surface behavior influenced by the neutral nature of CYM in deionized water.
Comparative analysis of the solid-state 13C NMR spectra of the four nanoparticles revealed distinct structural features (Fig. 3A). The MON-OH nanoparticles exhibited characteristic resonances at 43.19, 23.44, and 11.13 ppm, corresponding to the -CH2 groups in the framework structure. After functionalization with APTES, the MON-NH2 spectrum displayed shifts in the signals at 43.31, 22.21, and 11.05 ppm for the framework -CH2 groups, along with a newly emerged resonance at 59.74 ppm, which was attributed to the -CH2 moiety adjacent to the amino group [45]. These spectral changes confirmed the successful grafting of amine functionalities onto the MON-OH framework. Subsequent modification with SA produced characteristic resonances in the 140 − 110 ppm range, arising from the non-conjugated carbon atoms of SA. Notably, the appearance of a distinct peak at 165.38 ppm was assigned to the carbonyl carbon (C=O) of the newly formed amide bond, clearly demonstrating the covalent conjugation of SA to MON-NH2 through amide linkage formation [46]. Finally, the successful incorporation of cymoxanil was confirmed by the emergence of a characteristic nitrile (C≡N) resonance at 118.62 ppm in the CYM@MON-SA nanoparticles [47].
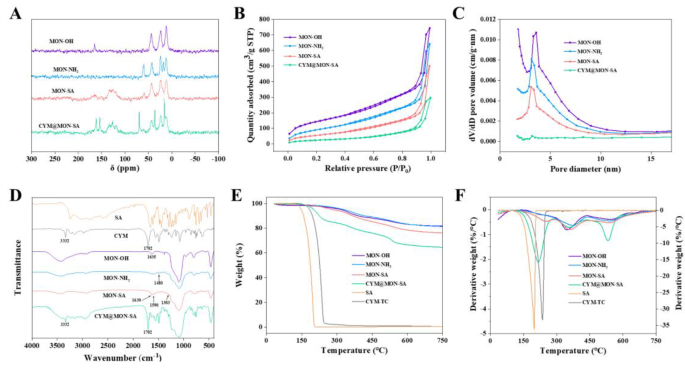
Characterization and properties of nanoparticles. (A) Solid-state 13C NMR spectra; (B) N2 adsorptiondesorption isotherms; (C) Pore size distribution from BJH adsorption; (D) FTIR spectra; (E) TGA curves; (F) DTG curves
Further physiochemical properties of the nanoparticles were thoroughly characterized. As shown in Figs. 3B and C, the BET and BJH analysis of the four nanoparticles were performed using nitrogen adsorption-desorption isotherms. MON-OH exhibited well-defined mesoporous nanopores with a specific surface area of 529.50 m2/g, a pore volume of 1.15 cm3/g, and a pore diameter of 5.41 nm. After treating with APTES and grafting salicyloyl chloride onto the NH2 group, the mesoporous framework was largely preserved, though there was a decrease in surface areas, with values of 342.34 m2/g and 207.74 m2/g, and a reduction in pore volumes to 0.99 cm3/g and 0.77 cm3/g for MON-NH2 and MON-SA, respectively. More importantly, after coating with CYM, the specific surface area, pore volume, and pore diameter of CYM@MON-SA were significantly reduced to 50.47 m2/g, 0.26 cm3/g, and 3.60 nm, respectively (Table 2). These results unequivocally demonstrate the successful encapsulation of SA and CYM within the MON-NH2 framework to form CYM@MON-SA [48].
The FTIR spectra (Fig. 3D) further confirmed the composition of these nanoparticles. In comparison with MON-OH, two new peaks at 1480 cm− 1 and 1570 cm− 1 corresponding to N-H stretching and bending vibrations were observed in MON-NH2, indicating the successful introduction of amino groups [49]. After grafting with SA, a characteristic C-OH (phenolic) stretching peak at 1303 cm− 1, a broader absorption peak at 1590 cm− 1 corresponding to C=C stretching vibrations of the benzene ring, and a peak at 1630 cm− 1 for the C=O stretching vibration of the amide bond, confirming that MON-NH2 had been grafted with SA via amide bonds [25]. Furthermore, two characteristic peaks at 3332 cm− 1 and 1702 cm− 1 were observed in CYM@MON-SA, attributed to the functional groups of the CYM active ingredient, confirming the successful encapsulation of CYM within the MON-SA framework.
Furthermore, TGA and DTG were performed for quantitative evaluation. As depicted in Fig. 3E, the weight loss of MON-OH was calculated to be 18.44%, which slightly increased to 18.74% after amino-functionalization to form MON-NH2. The total weight losses for MON-SA and CYM@MON-SA increased to 24.04% and 35.43%, respectively, due to the decomposition of SA and CYM. The DTG spectra (Fig. 3F) revealed a sharp peak at approximately 253 °C for MON-SA and at 218 °C for CYM@MON-SA, confirming the successful grafting of SA and the loading of CYM. Based on TGA data, the content of released SA in MON-SA was approximately 5.30%, while the released SA and loaded cymoxanil in CYM@MON-SA were about 4.70% and 11.39%, respectively (Table 2). These values were approximately consistent with the SA release (6.72%) from MON-SA and the SA release (6.08%) and CYM loading (12.27%) from CYM@MON-SA, as determined by HPLC (Table 2).
Stimuli-responsive properties and controlled release behaviors
As reported, the disulfide bonds (S-S) and amide bonds are susceptible to cleavage by glutathione (GSH) and amidase (AM), respectively [11, 22], which were substantially formed in CYM@MON-SA nanoparticles. To evaluate the response of CYM@MON-SA to these stimuli, the nanoparticles were exposed to H2O, and 8 mM GSH + 100 U/L AM in deionized water (pH = 7) [50, 51]. TEM images taken at various time points revealed progressive structural degradation with 8 mM GSH + 100 U/L AM, indicating that CYM@MON-SA nanoparticles can respond to both GSH and AM, enabling controlled release of SA and CYM (Fig. 4A-E). In contrast, H2O-treated nanoparticles maintained their structural integrity without observable morphological changes throughout the experimental period (Fig. 4A-E), demonstrating great stability in H2O (pH = 7). To further investigate the stability of nanoparticles under various conditions, subsequent studies could validate their performance across different aqueous media or through simulated foliar spraying applications.
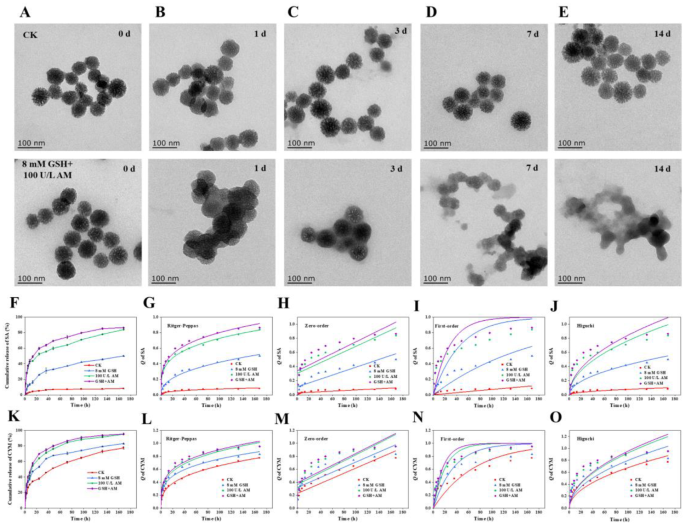
Stimuli-responsive properties and controlled release of CYM@MON-SA nanoparticles. Morphological degradation of CYM@MON-SA treated with H2O (used as the control, CK, pH = 7) or 8 mM glutathione (GSH) + 100 U/L amidase (AM) buffer (pH = 7) at (A) 0 d, (B) 1 d, (C) 3 d, (D) 7 d, and (E) 14 d; (F) SA release profiles under treatment (H2O, CK, pH = 7), 8 mM GSH (pH = 7), 100 U/L AM (pH = 7) and 8 mM GSH + 100 U/L AM (pH = 7), and their release kinetics analyzed by (G) Ritger-Peppas, (H) Zero-order, (I) First-order, and (J) Higuchi model; (K) CYM release profiles under treatment (CK, pH = 7), 8 mM GSH (pH = 7), 100 U/L AM (pH = 7) and 8 mM GSH + 100 U/L AM (pH = 7), and their release kinetics analyzed by (L) Ritger-Peppas, (M) Zero-order, (N) First-order, and (O) Higuchi model
Accordingly, we assessed the release profiles of SA and CYM under H2O, 8 mM GSH, 100 U/L AM, and 8 mM GSH + 100 U/L AM treatment. After 168 h at 25 °C, the cumulative release of SA from CYM@MON-SA under H2O, 8 mM GSH, 100 U/L AM, and 8 mM GSH + 100 U/L AM treatment was 8.15%, 50.05%, 83.94%, and 86.42%, respectively (Fig. 4F-J), while the cumulative release of CYM was 77.36%, 83.08%, 95.06%, and 95.30%, respectively (Fig. 4K-O). Furthermore, the release experiments in plant matrix or leaf extract can be carried out in the future to simulate the biochemical environment of the real world.
To gain deeper insights into the release mechanisms of SA and CYM under different conditions, the release kinetics were analyzed using Zero-order, First-order, Ritger-Peppas, and Higuchi models [52]. The results indicated that the release of SA and CYM from CYM@MON-SA under different conditions was best fitted to the Ritger-Peppas model, with R2 ranged from 0.93 ~ 0.99 (Fig. 4F-O; Table 3). According to the Ritger-Peppas model, the value of n provides insights into the release mechanism: n ≤ 0.43 indicates Fickian diffusion; 0.43 n n = 0.85 corresponds to Case II transport; and n > 0.85 represents Super Case II transport [52]. In this study, all n values were found to be less than 0.43, indicating that the release of SA and CYM from CYM@MON-SA nanoparticles under GSH and amidase treatments followed a Fickian diffusion mechanism. Collectively, these findings demonstrate that CYM@MON-SA nanoparticles exhibit a dual responsiveness to both GSH and amidase.
While this study employed literature-based concentrations of GSH and AM [35, 50, 51], subsequent studies should establish concentration-response release profiles for CYM@MON-SA nanoparticles, and specifically measure the dynamic changes of GSH and AM concentrations in cucumber leaves under different conditions (e.g., before and after pathogen infection). Such investigations would enable more in-depth research on the responsive release mechanisms of nano-fungicides at physiologically relevant concentrations, thereby better approximating field application scenarios.
Photostability of CYM@MON-SA nanoparticles
To investigate the effect of nanomaterial loading on the photostability of CYM, we measured the degradation rates of CYM-TC and CYM@MON-SA nanoparticles under UV light irradiation. After 3 h of exposure, CYM-TC degraded by 56.59%, whereas the degradation rate of CYM in CYM@MON-SA was only 23.01%. After 10 h, CYM-TC had degraded by 94.65%, while the degradation rate of CYM in CYM@MON-SA was 63.90%. After 24 h, CYM-TC showed a degradation rate of 99.66%, while CYM in CYM@MON-SA degraded by 78.08% (Fig. 5A). The photodegradation data were well fitted to First-order kinetic model (Fig. 5B). The photodegradation rate constants and half-lives were 0.25 h− 1 and 2.77 h for CYM-TC, and 0.08 h− 1 and 8.91 h for CYM@MON-SA, respectively (Table 4). The photodegradation half-life of CYM@MON-SA was extended by 3.22-fold relative to CYM-TC. These results clearly demonstrate that CYM@MON-SA significantly enhances the photostability of CYM. This improvement is primarily attributed to the protective effect of the nanoparticles, which absorb or reflect UV light, effectively preventing the photodegradation of CYM within the mesoporous channels of the MSNs [47]. However, these results were obtained under controlled laboratory conditions. Subsequent studies can evaluate photostability in real field environments to better simulate practical agricultural applications. Currently, only a limited number of CYM-loaded nanocarriers have been developed. Zhang et al. fabricated a non-phospholipid liposomal nanocarrier (CYM-loaded sterosomes) that obtained CYM release for 3 d [36], while they did not evaluate whether this formulation improved CYM’s photostability. In contrast, CYM@MON-SA nanosystem developed in our study effectively addresses the photolability issue of CYM.
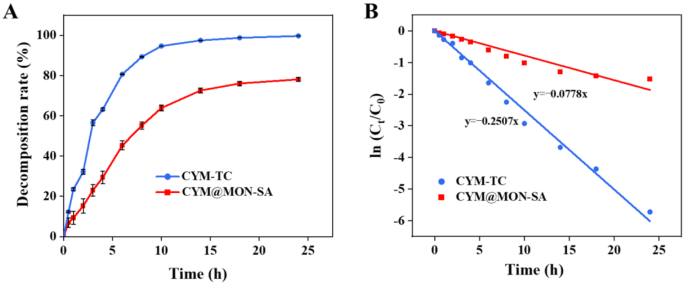
Photostability of CYM@MON-SA nanoparticles and CYM-TC under UV irradiation. (A) Decomposition rates and (B) First-order models for photodegradations of CYM@MON-SA nanoparticles and CYM-TC
Dynamic contact angle determination
The wettability and adhesion of fungicide droplets on plant leaves are key factors in improving deposition and optimizing effectiveness, and dynamic contact angle measurements are typically used to evaluate the interfacial adhesion of droplets on plant surfaces [53]. The contact angles of all samples on cucumber leaves were determined, which gradually decreased over time during the 0–120 s observation period (Fig. 6A). As observed, the contact angles of H2O (control), MON-NH2, MON-SA, CYM@MON-SA, SA and CYM-TC on cucumber leaves were 112.61°, 93.77°, 87.69°, 87.18°, 92.01° and 82.12° after 120 s (Fig. 6B), respectively. CYM-TC exhibited the smallest contact angle, demonstrating its inherent hydrophilicity and superior wettability. The results indicate that the wettability and affinity of the nanoparticles on cucumber leaves are improved due to the introduction of hydrophilic SA moieties. After functionalizing MON-NH2 with SA, the contact angle decreased significantly, suggesting that this modification might enhance the uptake of nanoparticles by cucumber plants and improve the utilization efficiency of SA [11, 54, 55].
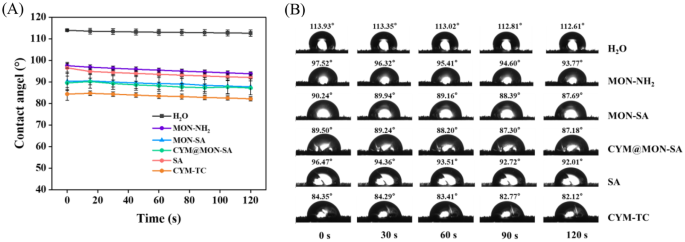
Quantitative wetting analysis of the nanoparticles on cucumber leaves. (A) Statistical analysis of contact angles; (B) Droplet morphology
Inhibitory activity of nanoparticles against P. cubensis
To date, the obligate biotrophic P. cubensis can only be cultured in vivo on cucumber plants and cannot be maintained in axenic culture on artificial media [33, 56]. Therefore, the fungicidal activities of nanoparticles against P. cubensis were evaluated using the cucumber leaf disc method. As shown in Figs. 7A and B, CYM@MON-SA, CYM-TC, CYM-SC, and CYM-TC + SA exhibited significant inhibitory activities against P. cubensis on cucumber leaf discs, with mean inhibition rates of 86.11%, 30.56%, 51.85%, and 25.70%, respectively. Compared to CYM-TC and CYM-SC, the inhibition rate of CYM@MON-SA was significantly increased by 55.56% and 34.26%, respectively, at the same CYM concentration of 400 µg/mL. This enhanced inhibition rate is primarily attributed to the improved photostability and controlled release of CYM from CYM@MON-SA, which increases the effective utilization of CYM.
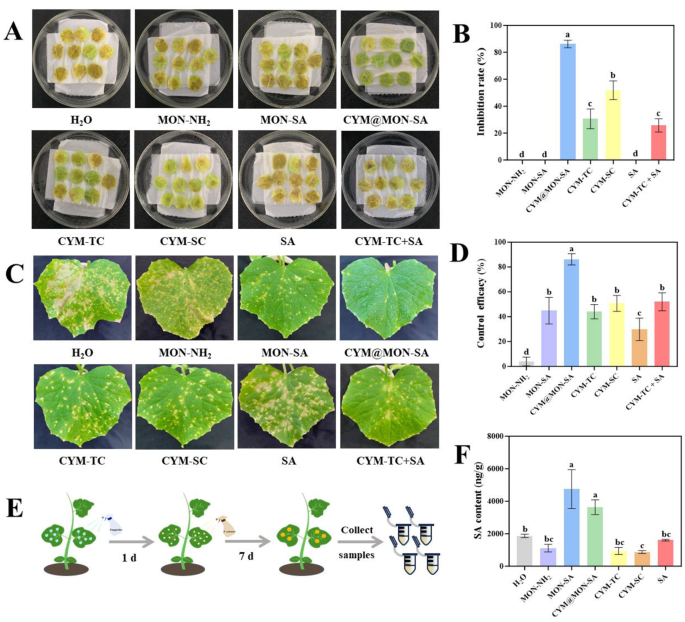
Determination of inhibitory activity, control efficacy and salicylic acid (SA) contents. (A) Images and (B) data statistics histogram of inhibitory activities of nanoparticles against Pseudoperonospora cubensis on cucumber leaf discs; (C) Images and (D) data statistics histogram of control efficacies of nanoparticles against cucumber downy mildew (CDM) on cucumber plants at corresponding final concentrations (CYM: 400 µg/mL, SA: 200 µg/mL); (E) Diagram of control efficacy determination and sample collection process; (F) Salicylic acid contents in collected samples. The “H2O” represents the control, in which cucumber seedlings were sprayed with 5 mL of deionized water containing 0.1% Tween 20 prior to P. cubensis inoculation. Columns represent the means, and the bars denote the standard deviations (SDs). Different letters above the columns indicate statistically significant differences (p
In contrast, MON-NH2, MON-SA and SA did not exhibit significant inhibitory activity against P. cubensis on cucumber leaf discs in vitro. This suggests that SA (at a concentration of 200 µg/mL) does not have substantial inhibitory activity against P. cubensis, likely due to its inability to effectively activate the systemic immune resistance on cucumber leaf discs in vitro. Similar results have been reported that exogenous application of SA on PDA plates showed no antifungal activity against Sclerotinia sclerotiorum [4], due to the failure to induce systemic acquired resistance (SAR) in plants, which is essential for pathogen defense. Additionally, a previous study demonstrated that CYM-loaded sterosomes prolonged antifungal activity against non-phytopathogenic Saccharomyces cerevisiae [36]. However, the CYM@MON-SA nanosystem developed in our study specifically targeted agriculturally relevant plant pathogen, demonstrating direct applicability in crop protection.
Control efficacy of nanoparticles against cucumber downy mildew
To further elucidate the control efficacies of nanoparticles against cucumber downy mildew (CDM) in cucumber plants, we sprayed the plants with MON-NH2, MON-SA, CYM@MON-SA, CYM-TC, CYM-SC, SA, and CYM-TC + SA, followed by inoculation with P. cubensis after 24 h. As shown in the Figs. 7C and D, the control efficacies of MON-NH2, MON-SA, CYM@MON-SA, CYM-TC, CYM-SC, SA, and CYM-TC + SA were 4.04%, 44.88%, 86.22%, 44.05%, 50.73%, 29.86%, and 51.98%, respectively, against CDM on cucumber plants. Compared to commercial CYM-SC, CYM@MON-SA exhibited a 35.49% significant increase in control efficacy, demonstrating superior performance against CDM. Compared to the direct mixture of CYM-TC and SA at the same concentration, the control efficacy of CYM@MON-SA was significantly increased by 34.24%. Additionally, based on the control efficacies of CYM-TC and SA alone, the theoretical control efficacy of this mixture was calculated to be 60.75% using the Colby formula [37]. The synergy factors (SFs) for CYM@MON-SA and the CYM-TC + SA mixture were 1.42 and 0.86, respectively, indicating that CYM@MON-SA exhibited synergism, while the direct mixture of CYM and SA showed antagonism [37, 38]. The observed synergism of CYM@MON-SA is likely due to the co-delivery and sustained release of CYM and SA, as well as the enhanced photostability of CYM.
Moreover, we found that the control efficacy of MON-SA was 28.35% higher than that of SA alone, suggesting that the nanocarriers may improve the characteristics of SA, such as facilitating slow release and prolonging its action in cucumber plants. To investigate this further, we collected cucumber leaves after treatment (Fig. 7E) and measured the SA contents. The results showed that the SA contents in MON-SA and CYM@MON-SA-treated plants were higher than those treated with MON-NH2, CYM and SA (Fig. 7F). It is speculated that the MON-SA and CYM@MON-SA nanoparticles facilitate the hydrolysis of amide bonds by plant-derived amidase for continuous release of SA to enhance the plant’s immune response, thereby working synergistically with CYM to resist P. cubensis.
CYM@MON-SA enhance the plant defense response against CDM
SA plays a crucial role in activating plant defense response to induce systemic acquired resistance (SAR) against biotic or abiotic stresses [55, 57, 58]. It is physically bonded by the soluble plant protein catalase (CAT) [59] and inhibits the H2O2 hydrolyzing activity of CAT [60]. To determine whether the improved efficacy of MON-SA and CYM@MON-SA nanoparticles is due to enhanced plant defense responses, we measured the CAT activity in cucumber leaves after treatment with the nanoparticles and P. cubensis inoculation. The results showed that the CAT activities in cucumber leaves under MON-SA and CYM@MON-SA treatments were 15.95 U•mg− 1•min− 1 and 10.42 U•mg− 1•min− 1, respectively, which were significantly lower than those with other treatments (Fig. 8A). Previous studies have shown that exogenous SA inhibits the CAT activity in plants [19, 61, 62]. Therefore, after the application of MON-SA and CYM@MON-SA, the inhibition of CAT activity is more pronounced due to the increase of SA content.
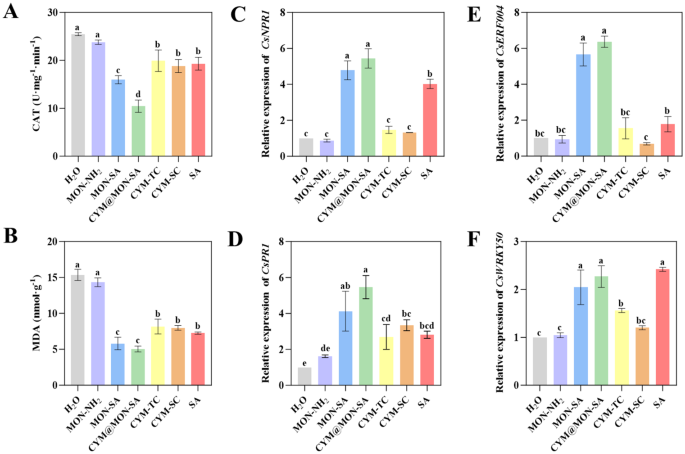
MON-SA and CYM@MON-SA nanoparticles enhance the plant defense response against cucumber downy mildew. (A) Catalase (CAT) activities; (B) Malondialdehyde (MDA) contents; (C) CsNPR1, (D) CsPR1, (E) CsERF004, and (F) CsWRKY50 resistance gene expression levels. The columns represent the means, and the bars denote the SDs. Different letters above the columns indicate statistically significant differences (p
In addition, SA can induce the changes of certain substances within the plant, such as malondialdehyde (MDA), which is a key indicator of the antioxidant capacity and oxidative damage extent of organism [18, 63]. We also measured the MDA contents in cucumber leaves with various treatments. As shown in Fig. 8B, compared to those with other treatments, the MDA contents in cucumber leaves treated with MON-SA and CYM@MON-SA were decreased significantly, with values of 5.81 nmol/g and 5.04 nmol/g, respectively. The results showed that the nanoparticles grafted with SA resisted the oxidative stress reaction of plants during the process of pathogenic infection, thus reducing the content of MDA, a natural product of lipid oxidation.
Most importantly, SA resists pathogen stress by activating the expression of defense genes, including pathogenesis-related (PR) genes [58, 64]. PR genes were also activated by non-expresser of pathogenesis-related protein 1 (NPR1), a downstream receptor of SA [65, 66], and the overexpression of NPR1 gene results in enhanced disease resistance in plants [57, 67]. Additionally, the upregulation expression of resistance disease genes also enhances cucumber resistance to P. cubensis, like CsERF004 [68] and CsWRKY50 [69]. To determine whether the improved efficacy of MON-SA and CYM@MON-SA nanoparticles is due to activate the expression of disease resistance genes, we measured the expression levels of four disease resistance genes (CsNPR1, CsPR1, CsERF004 and CsWRKY50) in cucumber leaves with various treatments. The results showed that the expression levels of CsNPR1, CsPR1 and CsERF004 in cucumber leaves under MON-SA and CYM@MON-SA treatments were higher than those with other treatments (Fig. 8C-E). While the expression level of CsWRKY50 under MON-SA and CYM@MON-SA treatments was comparable to that of the direct SA spraying treatment, all of which were higher than those in other treatments (Fig. 8F). The results suggest that MON-SA and CYM@MON-SA nanoparticles could more strongly and persistently activate plant defense responses to enhance resistance to P. cubensis.
Biosafety of CYM@MON-SA nanoparticles to cucumber plants
As P. cubensis exclusively infects cucumber leaves, conventional control strategies in agricultural practice primarily involve foliar spray of fungicides [34, 56]. Therefore, to assess the safety of nanoparticles spraying on plants, we measured changes in plant height and stem diameter of cucumber plants 7 d and 14 d after nanoparticle application. As shown in Fig. 9, on the 7th day after spraying, the plant height with CYM@MON-SA treatment increased by 16.45 mm, and the stem diameter increased by 0.80 mm. On the 14th day post-spraying, the plant height with CYM@MON-SA treatment increased by 71.80 mm and the stem diameter increased by 1.47 mm. The results showed that there were no significant differences in leaf color, plant height, and stem diameter between the CYM@MON-SA nanoparticle and the other treatments, indicating that CYM@MON-SA exhibited desirable biosafety for cucumber plants under the experimental concentration range. Previous studies have demonstrated that SA can regulate the plant microbiome by increasing microbial diversity, maintaining core microbial populations and promoting the colonization of rare satellite taxa, thereby benefiting host plants [70, 71]. Future studies could further investigate the effects of nanoparticles on the phyllosphere microbiome through metagenomic sequencing to better understand their bioactivity and biosafety.
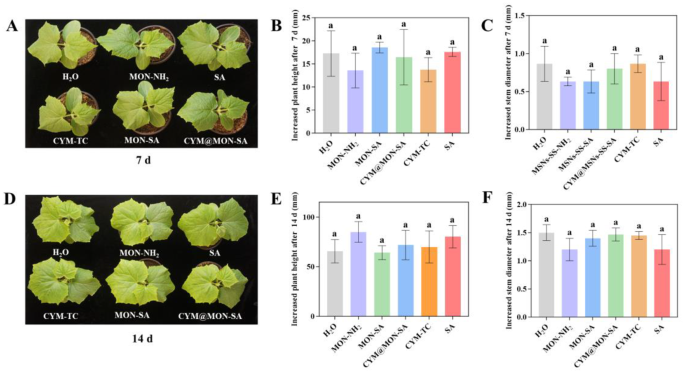
Biosafety of nanoparticles in cucumber plants. (A) Images, (B) increased height, and (C) stem diameter of cucumber plants after 7 d of treatments. (D) Images, (E) increased height, and (F) stem diameter of cucumber plants after 14 d of treatments. The columns represent the means, and the bars denote the SDs. Different letters above the columns indicate statistically significant differences (p


